Continuous Cell Culture Operation at 2,000-L Scale
 In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (1). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture, harvest, and product purification must be effectively integrated (2). That follows a trend within the industry to take an increasingly holistic view of bioprocesses and bioprocess platforms encompassing seed bioreactors through downstream processing to drug substance freezing and thawing.
In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (1). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture, harvest, and product purification must be effectively integrated (2). That follows a trend within the industry to take an increasingly holistic view of bioprocesses and bioprocess platforms encompassing seed bioreactors through downstream processing to drug substance freezing and thawing.
Cell culture operations play a vital role in determining overall process productivity. The industry has made great progress over the past couple decades in achieving higher product titers from fed-batch cell cultures. One consequence has been a move toward smaller bioreactors and replacement of older stainless steel equipment with single-use technologies. Some companies are choosing to develop perfusion processes. They are especially useful when a therapeutic product is labile and has a short halflife within a production bioreactor.
Under perfusion control, protein can be constantly harvested from a culture vessel and purified before degradation occurs. The constant addition of nutrients and removal of toxic metabolites allows perfusion cultures to reach and sustain high cell densities over many weeks. Not only is that a highly productive condition, but it also allows cells to remain within a steady state and express consistent product. Advances in cell culture media and improvements in the robustness of microfilter cell-retention devices have contributed to the adoption of perfusion.
Some disadvantages exist, however, including challenges in operating cell cultures for prolonged periods: e.g., preventing contamination and demonstrating consistency during validation from harvest and purification of multiple “subbatches.” Those concerns are particularly important during drug development for companies trying to rapidly reach their investigational new drug (IND) filing. So some companies are deciding to operate in perfusion mode, through which product is retained (sometimes called concentrated fed-batch mode) as fresh media is added and exhausted media removed using an ultrafiltration device. Very high cell densities can be generated and maintained that give exceptional productivity in a short time frame without the additional complexity of harvesting and purifying multiple “sub-batches” of product.
We believe that the full potential of continuous upstream operations has yet to be realized. Applications of new technologies can help deliver upstream bioprocesses with even higher productivities at preclinical, clinical, and commercial manufacturing scales. With such high titers, even blockbuster drugs could be manufactured using 2,000-L single-use bioreactors, and no additional scale-up step after process validation in phase 3 would be needed. That offers the advantage of omitting an additional scale-up step and eliminating the associated time and risk of further adapting a bioprocess. In addition, drug makers can exploit all the advantages of single-use manufacturing through perfusion operations.
Concentrated Fed-Batch or Perfusion Operations: After inoculation of a bioreactor and an initial batch growth phase, removal of cell-free supernatant using a cell retention device begins at a constant harvest flow rate. Meanwhile, the culture is replenished with an equivalent volume of fresh medium. Control of its addition can be achieved through a feed pump that receives a signal from load cells or a platform balance that enables operators to maintain a defined bioreactor weight. Multiple approaches might be used, including non-automated daily feed rate adjustments based on nutrient levels.
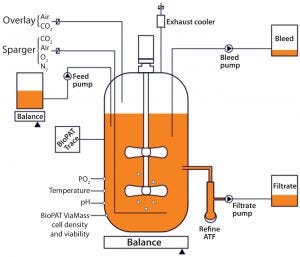
Figure 1: Set-up of a concentrated perfusion or fed-batch set-up using the Biostat STR single-use bioreactor
As cell density grows and nutrient consumption and metabolite formation increase, the exchange rate increases to provide a certain amount of fresh medium per cell per day — or alternatively, to follow a predefined profile of medium exchange per day (3). On-line biomass measurement provides an automated option to control perfusion or cell bleed rate based on a cell-density signal. Using an additional concentrated feed can help operators control the glucose concentration in a bioreactor using feedback from at-line glucose and lactate measurements. The schematic in Figure 1 depicts a typical concentrated perfusion or fed-batch set-up.
Key Considerations: Typical perfusion rates fall into the range of one to two bioreactor volumes per day. Applying a small cell-bleed stream enables establishment of a defined cell growth rate, and by that a high viability can be maintained that in turn mitigates clogging of the cell retention device (4). Depending on the pore size or cut-off of the cell retention membrane, a protein product will be either recovered in the cell-free harvest (microfiltration perfusion) or retained in the bioreactor (ultrafiltration perfusion).
Most antibodies are relatively stable, so perfusion with accumulation of such products in a bioreactor offers a simple and straightforward approach to increase the space/time yield of a given antibody-manufacturing facility. Microfiltration perfusion is the method of choice for recombinant proteins that are prone to degradation. And those that might show feedback inhibition should be removed from cell culture as fast as possible and transferred into a chilled harvest tank before subsequent purification.
Using Sartorius stirred-tank, single-use bioreactors in combination with different sizes of cell retention devices, perfusion processes can be developed at the 2-L bench scale. For example, a company can use a Univessel single-use bioreactor in combination with a Biostat B or B-DCU controller and subsequently scale its process to 500 L, then 1,000 L in a Biostat STR system. At 1,000-L and 2,000-L scales, a retention device (e.g., ATF system filter modules from Repligen) connects through side ports of a single-use bioreactor bag with up to two 1-in. sterile connectors and operates in an external loop of the bioreactor.
Alternatively, Spectrum Labs and other companies propose using single-use, hollow-fiber modules operated in tangential-flow mode for cell retention. Another cell retention option is to use a continuous centrifuge such as a kSep single-use centrifuge from Sartorius or a Carr Centritech Cell system from PneumaticScaleAngelus. The advantage of continuous centrifugation is that it eliminates filter clogging and allows dead cells to be selectively separated and removed depending on the chosen centrifugal force.
For all those options, it is critical that the external loop be as short as possible to keep from exposing cell cultures to uncontrolled conditions, most critically those related to temperature and dissolved oxygen (DO).
Single-Use Bioreactor Configurations Suitable for Intensified Cell Cultures: The key to successful perfusion operations is an efficient aeration system that provides kLa values above 10–15 h–1 to supply cultures with sufficient oxygen. During an intensified culture run, large amounts of carbon dioxide are formed and must be removed to prevent inhibitory effects on productivity or possible negative effects on product quality.
With Biostat STR single-use bioreactors, that is achieved with a combination sparger that “microsparges” compressed air or pure oxygen through defined 150-µm holes while providing a stripping gas flow through 0.8-mm holes simultaneously. This single-use sparger design emulates a successful aeration strategy that has been used for many years in conventional stainless steel bioreactors.
A problem that should not be underestimated is excessive aerosol formation in exhaust gas due to high gas-flow rates and protein contents in concentrated cell cultures. A specifically developed single-use exhaust condenser design based on the well-known principle of plate heat exchangers can reduce the risk of blocked filters and dramatically increase process reliability. Additional safety interlocks in Sartorius bioreactor-control software prevent overflow in the event of a clogged cell retention device. Furthermore, all feed pumps and gas flows are interrupted if pressure in a Biostat STR bioreactor exceeds its maximum defined operating pressure.
Here, we explore the use of this 2,000-L single-use bioreactor for production of two different monoclonal antibodies (MAbs) by continuous culture.
Methods
A large US biotech company evaluated the performance of the new system against that of existing single-use and stainless steel bioreactors in two perfusion processes. Both processes used a common cell-retention device with Chinese hamster ovary (CHO) cell lines to produce two different MAbs.
With a 2,000-L maximum working volume, the 2000 is the largest model in the Biostat STR range of single-use bioreactors from Sartorius Stedim Biotech. Low-shear agitation with three-blade segment impellers provides homogeneous mixing. A microsparger designed not to shed particles has defined 150-µm holes to allow oxygen transfer rates of ≤40 h–1. A classical stirred-tank design enables application of well-known and established scale-up criteria such as power-input per unit volume or tip speed. Geometric similarity from the ambr 250 microbioreactor (with its working volume of just 250 mL) across the Sartorius range of single-use bioreactors to this 2,000-L unit allows for seamless scale-up (5).
Bags for Biostat STR bioreactors are made of the new S80 multilayer polyethylene film, recently introduced by Sartorius for the Flexsafe range of bioprocess containers. Biomanufacturers can use the same film for storage, and it will be available in the future also for mixing, shipping, and freeze–thaw applications. That will help companies reduce the number of materials their products will come into contact with from beginning to end of their manufacturing processes.
In the context of cell culture, S80 film delivers reproducible cell growth comparable to that of borosilicate glass (6, 7). It is physically robust as measured by tensile strength and other test methods and adheres to the ASTM D4169-09 guideline for shipping applications (8). Flexsafe bags are manufactured with a holistic strategy that takes consistent quality, assurance of supply, change control, and business continuity into consideration (9). This bioreactor (including consumables) has been verified at the 1,000-L scale using a CHO fed-batch MAb production process (10).
To benchmark the system and demonstrate its functional equivalence with an existing single-use bioreactor, the company used a continuous cell culture process for production of MAb A. We compared data from four Biostat STR 2000 runs with historical data averaging eight previous runs in the existing bioreactor.
To test the new system’s capabilities, we also compared its performance with that of the existing single-use and a stainless steel bioreactor for production of MAb B. This is a more demanding process than what was used to produce MAb A because it generates higher cell densities that demand more oxygen. Such conditions can be challenging for single-use bioreactors, which are typically not designed to handle exhaust gases from such intense processes.
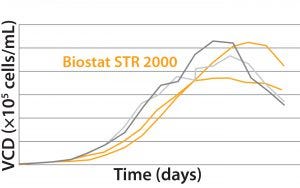
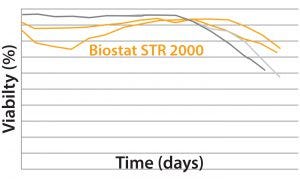
Results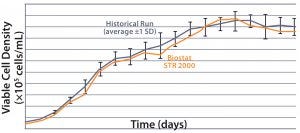 MAb A — Bioreactor Functional Equivalence Benchmarking: Figure 2 compares viable cell density (VCD) and cell viability (%) data from a Biostat STR 2000 system with historical data
MAb A — Bioreactor Functional Equivalence Benchmarking: Figure 2 compares viable cell density (VCD) and cell viability (%) data from a Biostat STR 2000 system with historical data
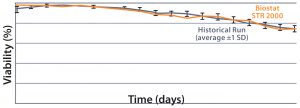
Figure 2: Comparing viable cell density (top) and cell viability (bottom) over time from a Biostat STR 2000 bioreactor with historical data generated from an existing single-use bioreactor for the MAb A process.
generated from an existing single-use bioreactor. Averaged data from the former falls within one standard deviation of the mean generated from those historical data.
Figure 3(top) compares the packed-cell volume data (%) from both bioreactors. Results show a high degree of comparability between the two systems. Figure 3(bottom) compares their packed-cell adjusted product titers, and once again the two systems are comparable. From 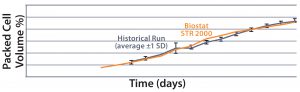 these results, we can conclude that the new system is functionally equivalent to the 2,000-L single-use bioreactor already in use. Here, we observed that less oxygen was needed to maintain the DO set
these results, we can conclude that the new system is functionally equivalent to the 2,000-L single-use bioreactor already in use. Here, we observed that less oxygen was needed to maintain the DO set
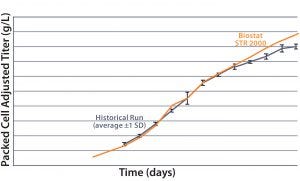
Figure 3: Comparing the percentage of packed cell volume data and packed-cell adjusted product titer over time from a Biostat STR 2000 with historical data generated from an existing single-use bioreactor for the MAb A process
point of the culture for the new system compared with historical data, which suggests a better oxygen transfer capability (not shown).
MAb B — Testing Biostat STR with a More Challenging Process: Two different MAb B production processes were run in a Biostat STR 2000 system. One process takes 25% longer than the other to run. 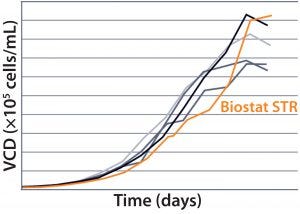 For the shorter process, VCD and percent viability were comparable with those of historical data from the existing single-use bioreactor (Figure 4). Packed-cell adjusted product titer data also were comparable (not shown). However, a significant difference was observed between the two 2,000-L single-use bioreactors during this shorter process: Total gas flow and bag
For the shorter process, VCD and percent viability were comparable with those of historical data from the existing single-use bioreactor (Figure 4). Packed-cell adjusted product titer data also were comparable (not shown). However, a significant difference was observed between the two 2,000-L single-use bioreactors during this shorter process: Total gas flow and bag
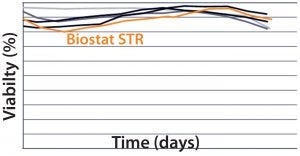
Figure 4: Viable cell density (VCD) and viability (%) over time were comparable with historical data from the existing single-use bioreactor for the MAb short B process.
pressure typically were lower in the new system than in the other single-use bioreactor. Apparently, oxygen transfer within the former is particularly efficient. Its lower gas flow rates minimize shear damage from bubble bursting and lowers the risk of exhaust-filter blocking due to foaming. That risk is mitigated further by the new bioreactor’s large headspace.
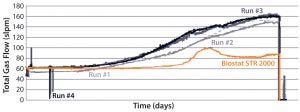 Figure 5 illustrates the difference in aeration performance between the two single-use bioreactors. These findings raised the possibility that MAb B could be manufactured in a Biostat STR 2000 system with the
Figure 5 illustrates the difference in aeration performance between the two single-use bioreactors. These findings raised the possibility that MAb B could be manufactured in a Biostat STR 2000 system with the
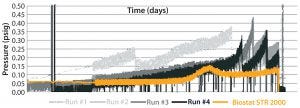
Figure 5: Difference in aeration performance between a Biostat STR 2000 bioreactor and an existing single-use bioreactor for the short MAb B process
longer process. Historically that was possible only in a stainless steel bioreactor because of limitations in oxygen transfer with single-use bioreactors that had been previously assessed from other suppliers.
 A second set of culture runs compared the Biostat STR 2000 system with a longer stainless steel bioreactor reference process. Figures 6 and 7 show that comparable performance was achieved. The lower total gas flow rates provided by the single-use bioreactor were sufficient to support
A second set of culture runs compared the Biostat STR 2000 system with a longer stainless steel bioreactor reference process. Figures 6 and 7 show that comparable performance was achieved. The lower total gas flow rates provided by the single-use bioreactor were sufficient to support
Figures 6: Viable cell density (VCD) and viability (%) over time were comparable with historical data from the stainless steel bioreactor for the long MAb B process.
cell growth and product formation, demonstrating the system’s superior oxygen transfer. Packed-cell adjusted product titer data also were comparable (not shown). Figure 6 also shows performance of the single-use system at two different agitation rates. Clearly the oxygen transfer rate need not be a limiting parameter with that bioreactor, thereby opening the door to longer processes, higher cell densities. and even greater productivity.
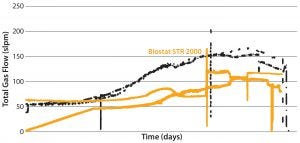
Figure 7: Difference in aeration performance between a Biostat STR 2000 bioreactor and a stainless steel bioreactor for the long MAb B process
As Good As or Better Than
We have demonstrated that the Biostat STR 2000 system provides equivalent performance to that of an existing single-use bioreactor for continuous production of MAb A. However, because of its superior oxygen transfer rates, less gassing is needed. That reduces the risk of exhaust-filter blockage from excessive foaming and pressure build-up in the bioreactor.
In a more demanding process for a second antibody, that superior oxygen transfer efficiency allowed a culture to run for a longer time than was achieved previously with single-use bioreactors. Process performance of the new system was comparable to that of a stainless steel reference process. So with a Biostat STR 2000 system, even higher cell densities and more MAb product can be generated from a single continuous run while retaining all the benefits of single-use processing.
Newly emerging technologies should enhance cell culture process development, scale-up, and process control significantly. Once these are applied in combination with high-performing, single-use bioreactors, then extremely high cell densities will become a reality. Control of critical process parameters requires advanced process control software and appropriate process analyzers. Single-use, precalibrated, and presterilized sensors are available for measurement of pH and DO. Oxygen and carbon dioxide sensors can be used in bioreactor exhaust lines both to monitor and provide additional insight into cell culture metabolism. Measurement of nutrients and metabolites such as glucose and lactate can be used during continuous cell cultures to provide information about nutrient supplies and the general metabolic state of a culture. That information can help companies determine the most appropriate nutrient feeding rates using fully automated control loops.
Automated viable-biomass measurements (e.g., those provided by BioPAT ViaMass sensors from Sartorius Stedim Biotech) can be completely integrated into single-use bioreactors to provide operator-independent information on viable biomass. That reduces the need for manual sampling through direct in-line measurements. By incorporating such sensors into a bioreactor control system, automatic monitoring and control of viable biomass will be feasible for continuous bioprocesses.
Bioreactor and cell culture technology continue to evolve, allowing ever higher cell densities to be achieved and maintained. Whether biopharmaceutical companies choose to work with fed-batch processes or develop continuous cell culture methods will depend on their individual circumstances. However technological innovations will allow them to enhance their speed to clinical testing and develop robust high-titer processes that operate within predefined design spaces.
References
1 Croughan M, Konstantinov KB, Cooney CL. The Future of Industrial Bioprocessing: Batch or Continuous? Biotechnol. Bioeng. 112(4) 2015: 648–651.
2 Warikoo V, et al. Integrated Continuous Production of Recombinant Therapeutic Proteins. Biotechnol. Bioeng. 109(12) 2012: 3018–3029.
3 Adams T, et al. Increasing Efficiency in Protein Supply and Cell Production By Combining Single-Use Bioreactor Technology and Perfusion. BioPharm Int. May 2011: S4–S11.
4 Fenge C, et al. Evaluation of a Spin Filter During Perfusion Culture of Recombinant CHO Cells. Animal Cell Technology: Development, Processes, and Products. Butterworth-Heinemann: Oxford, UK, 1992; 365–370.
5 De Wilde D, et al. Superior Scalability of Single-Use Bioreactors. BioProcess Int. 12(8) 2014: S14–S19.
6 Hammond M, et al. A Cytotoxic Leachable Compound from Single-Use Bioprocess Equipment That Causes Poor Cell Growth Performance. Biotechnol. Prog. 30, 2014: 332–337.
7 Fenge C, et al. Consistently Superior Cell Growth Achieved with New Polyethylene Film Formulation. BioProcess Int. 12(8) 2014: S34– S37.
8 Vachette E, et al. Robust and Convenient Single-Use Processing: The Superior Strength and Flexibility of Flexsafe Bags. BioProcess Int. 12(8) 2014: S38–S42.
9 Cappia JM, et al. Enhanced Assurance of Supply for Single-Use Bags. BioProcess Int. 12(8) 2014: S14–S19.
10 Reglin R, et al. Verification of New Flexsafe STR Single-Use Bioreactor Bags Using a CHO Fed-Batch Monoclonal Antibody Production Process at 1,000-L Scale. BioProcess Int. 12(8) 2014: S53–S57.
Michael Sherman was a applications specialist; Vincent Lam and Melissa Carpio are applications specialists; Nick Hutchinson is a technical writer; and corresponding author Christel Fenge, PhD, is vice president of marketing fermentation technologies, all in fermentation technologies at Sartorius Stedim Biotech GmbH, August-Spindler-Straße 11, DE-37079 Goettingen, Germany; [email protected].
You May Also Like





