Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

www.sartorius.com
After over a quarter century in the industry — including downstream processing (DSP) and manufacturing directorships at Boehringer Ingelheim and leadership roles in technology development, quality, and manufacturing at Novasep — European consultant Margit Holzer is a recognized expert in downstream processing of biopharmaceutical products. Holding a doctorate in biotechnology from the University of Natural Resources and Applied Life Sciences in Austria, Holzer is familiar to BPI readers as both an author and conference participant (1, 2). And in May she taught her first BPI Academy course on bioprocess characterization, qualification, and validation. Here we discuss membrane adsorbers, high-throughput screening, modeling and analytics, and monoclonal antibodies (MAbs) as vanguard products for advancing tools and technologies.
Single-Use Systems
How can single-use technologies such as membrane adsorbers contribute to a speed-to-IND strategy? Single-use materials have been used for early stage development in research laboratories for quite some time. Membrane adsorbers and single-use chromatography columns are recent developments. When needed product quantities are low and target recovery and confirmation times are key for companies, the move to single-use systems has been logical. Because research laboratories are accustomed to disposable materials such as pipettes and tubing, they have been amenable to acceptance of single-use filters, membrane adsorbers, and chromatography columns. Some new technologies such as spin-filters for product concentration or diafiltration also help to simplify downstream operations.
Single-use features that help to speed up product recovery for initial target design and confirmation, tests in animals, and in vitro studies include ease of use (most come as ready-to-use materials), elimination of carryover concerns, and control of microbial contamination. Single-use materials and their packaging are designed for use in ISO-standard laboratories.
Note that at early stages of development, scalability is seldom a concern. However, it does become important as soon as preparations for toxicological material are considered.
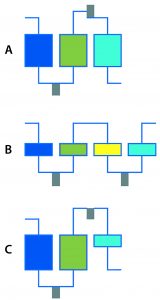
Figure 1: Multistep DSP examples; (a) Three-column process with integrated reaction and process-stream conditioning; (b) four membrane adsorbers with integrated reaction and process-stream conditioning; (c) three-step process with two columns, one membrane adsorber, integrated reaction, and process-stream conditioning
Automation
I’ve seen some impressive shortening of upstream timelines through automated clone picking and microscale bioreactors. How are such technologies changing the game in DSP? Quite some progress has been made based on automation in DSP as well. Systems for multistep processing are just such an advancement (Figure 1). They enable, for example, loading of harvested broth on one column with recovery of nearly pure material at the outlet of the multistep DSP. Purification of target products should be possible with a platform process so long as the composition of the harvested culture broth does not vary too much. Equipment for multistep processing needs additional features such as automated controls and triggers that enable product collection and preparation for the next steps in DSP: product conditioning (e.g., pH adjustments and dilutions), in-line filtration, and intermediate product holding for a defined residence time under certain conditions.
Is automation vital to making continuous chromatography possible? Absolutely, automated equipment is mandatory for continuous chromatography. Without “orchestrating” valves and instruments of several columns (see above), continuous operations would not be possible. This is also the case for the above described multistep DSP systems. Systems allowing automated screening of media and buffer solutions now are available not only for column chromatography, but also for membrane-based processes. Another advancement in this field is the fact that several columns, membranes, or filter units can be tested in parallel. Selected screen conditions are based on knowledge of the product, impurities, and materials.
High-Throughput Screening (HTS)
Similarly, we’ve seen high-throughput approaches for screening media formulations upstream. What can HTS do for downstream development — e.g., chromatographic media selection? HTS systems also have been adapted for DSP, with different formats for screening of chromatographic media, membranes, and filters. Such formats include 96-well filter plates, pipette tips, and miniature columns. The filter plates and pipette tips typically require only 50–100 µL of media and a similar amount of sample volume, which allows for reducing sample volumes per test condition. That is also the case for membrane adsorbers: for example, with a surface of 3 × 0.7 cm2 of membrane per well for separation (3).

Repligen’s OPUS RoboColumns miniature chromatography columns on Tecan’s Fluent laboratory automation solution
(www.youtube.com/watch?v=oXa_jZoXoTQ)
In 96-well filter plates, a sample containing the target protein is mixed or aspirated with a resin for binding, and elution is based on using different pH levels or salt concentrations. The flow-through material and eluates can be collected using vacuum or centrifugation processes while leaving the resin in the filter plate. For pipette tips, different loads and buffers pass through the device, and fractions are collected at the tip outlet. Columns used for robotic HTS have 5-mm internal diameters and up to 30-mm heights, using media volumes typically in the range of 100–600 µL.
To choose the right format and device, it is important first to define the objective of a screening, development, or characterization study. Objectives might be to gain a preliminary understanding of adsorption and desorption conditions or of correlations between process ranges and quality attributes or step yields. In one comparison of different HTP platforms using a protein A chromatography monoclonal antibody (MAb) capture process, a developer concluded that high-level scientific understanding for product yield and monomer purity could be gained from all the formats described above (4).
Combining robotic liquid-handling platforms such as Tecan and PerkinElmer liquid handlers with additional support tools enabling use of the screening devices described above results in an impressive study throughput. Note that specific chromatography or membrane process methods need to be programmed into a robotic liquid-handling system. It’s critical to maintain separate liquid streams, buffers, and samples or fractions to prevent cross contamination. Mobile robots that move 96-well plates between instruments could increase throughput even further (5).
Process Analytical Technology (PAT)
Is on-the-floor/in-process (at/on-line) testing viable for speeding up DSP? What difference can it make in timelines? And is there room for improvement in such technologies? Standard process-control instrumentation for parameters such as pH, conductivity, temperature, and pressure are important elements for screening systems. However, process-related impurities such as host-cell proteins (HCPs), DNA, and product-related substance concentrations cannot be measured automatically yet. HTP platforms involve a large number of very low-volume samples, so most quality attributes need to be analyzed with off-line methods that use minor quantities of samples and allow for parallel analyses.
Some companies also use HTP platforms for sample analysis. On-line UV2-wavelength or photodiode array (PDA) detectors can provide information about product and impurity concentrations based on different absorption patterns. Other spectroscopic methods such as Raman spectrometry seem to have the potential for application as PATs for indicating product and impurity concentrations in downstream development. Progress has been made toward use of such on-line methods in upstream processes (6), and several companies and universities are studying transfer of such technologies to DSP.
Mathematical Models: In addition to all the physical models, controls, and automation that have been developed, it also is important to mention the development of mathematical models such as software-based mechanistic process models that describe physical, chemical, and biological mechanisms. They can use data from predefined screening conditions to predict others. Optimization algorithms could help developers find process conditions for target product characteristics and productivities. Statistical models could be used to speed up this initial development work. They can be created based on data from design of experiments (DoE) testing conditions. A maximum benefit in product understanding and development time reduction could be achieved in the future if those different models are used together.
Platforms and Prior Knowledge
Even as new methods and approaches are changing things, prior-knowledge assessments remain important. What kinds of downstream platform approaches help to speed things along? Collecting prior knowledge about a target molecule and its physicochemical and structural properties together with information about the related impurity matrix is a starting point for downstream development. The term platform might be defined differently for each company and at each stage of development. But establishing filtration-process platforms for bioburden reduction and potentially for viral clearance and heat- treatment processes for viral inactivation can provide universal approaches for different products and cell-culture–derived broths in DSP.
For target molecules with similar characteristics (e.g., “standard” MAbs), the platform approach can be extended to a capture step usually accomplished with protein A affinity chromatography. Knowledge about stationary phases, a given MAb, and impurity profiles in the capture eluate are key factors affecting selection of conditions for intermediate purification and polishing steps. With novel molecules, we often can capitalize a great deal on our knowledge of process-related impurities such as HCPs, DNA, and media components.
Crossfunctional Teams
Can it help to include upstream experts in downstream decision-making — and vice versa? What other functions can contribute to downstream development efficiency? Crossfunctional teams bring enormous benefit to development projects, especially in preventing bad surprises such as changes in upstream conditions that make DSP results obsolete. Putting together such teams requires specialized training or expertise and an open mindset focused on project goals. Discussions and exchanges among team members with different knowledge and viewpoints also can take more time. Analytical experts are key team members for help in DSP: Without an understanding of analytical method capabilities, our interpretation of DSP studies can be inaccurate. Formulation experts need to be included in the team as well. And last but not least, clinical and regulatory team members are essential to guiding development work.
Quality By Design (QbD)
Many companies wait to implement QbD later in product development — e.g., during clinical testing, when good manufacturing practices (GMPs) must apply. But can it help to include risk assessment and identify quality attributes in early development — or does that inevitably slow things down? Pillars of QbD such as knowledge and risk management are applied more and more in a structured form during early stage development of new, second-generation, and biosimilar products. Furthermore, down-scaled model justification and risk analyses for viral clearance studies are obligatory, especially for products derived from mammalian cell culture, and necessary to meet safety requirements before products can be used in clinical trials. According to many companies’ guidelines, technology transfer from a non-GMP to a GMP environment must be supported by risk assessment. DSP unit-operation scale-down models can speed up the development phases that follow. QbD asks for a targeted, structured, and scientifically justified approach to early stage development but does not necessarily add additional work to a downstream development program.
Outsourcing
What aspects of working with contract development and manufacturing organizations (CDMOs) can slow things down in downstream development? And how can sponsors mitigate those problems? In general, working with CDMOs can speed up project development; in some cases that might slow it down. Additional interfaces add risks of information exchange, cultural and language differences, shipment delays, and so on. Note that slowing down at a certain project phases — e.g., because additional information or different studies are asked for — can be beneficial for a project in the long run. Key factors for reaching predefined “SMART” objectives include the experience, knowledge, and assets of a CDMO for DSP development; the capacity of both parties working in an open-exchange culture with crossfunctional teams from different companies; and application of solid project-management principles.
Characterization
How do analytics and early product-characterization efforts (e.g., HCP profiles and product-variant analysis) help move downstream development along? Product characterization is key to early stage DSP development. Initial stability studies of a drug substance can help us to understand process conditions that might, for instance, increase its aggregation, deamidation, or oxidation. In early DSP, analytical methods might show higher variability. Understanding variabilities in the product, analytical methods, and manufacturing process will be essential to determining whether a drug substance can be used for preclinical studies and to defining its readiness for process transfer into GMP production. Characterization data also are needed to define future DSP development plans and milestones.
Caveats
When quality and safety are at stake, it can be a mistake to focus only on speed. How might you caution others when it comes to shortening downstream process development timelines? If safety criteria or analytical capabilities are not reached, there is no way to shorten DSP development times and use material from such processes for toxicological studies or human treatment. Sometimes it might be more difficult to define the exact product-quality profile that should be used in preclinical studies. Medical experts must be involved in such evaluations. Even for them, linking, e.g., certain product forms with product efficacy might be difficult early on. Here, risk assessment can help to define target product-quality criteria and determine whether materials can be used.
Many tools and methods have been developed to help speed up DSP development to the IND application stage, especially for MAb products. Transitioning of such technologies to other types of biopharmaceutical products will be necessary.
Available single-use technologies for parallel testing of DSP unit operations already are a great help — as is the possibility of performing integrated multistep DSP development. But some companies ask whether investment in costly HTP robots, analytics, and resources is really necessary to perform “speedy” DSP development.
We should not use those technologies without careful thinking. It could be tempting to run a lot of experiments because “the robots will do the work for us.” But before anything else, a developer always should analyze available information, then clearly define objectives and use as little testing as possible — but perform the right tests!
A more efficient approach to DSP development might begin with improved experimental designs based on prior knowledge coupled with statistical, mechanical, and even molecular modeling. Together with PAT integrated into DSP equipment for parallel screening, data treatment and platforms for knowledge management could provide real-time product-quality information. That could be the next step in the future of DSP development.
References
1 Holzer M. Is Continuous Downstream Processing Becoming a Reality? BioProcess Int. May 2017; https://bioprocessintl.com/manufacturing/continuous-bioprocessing/continuous-downstream-processing-becoming-reality.
2 Montgomery SA, et al. Making Downstream Processing Continuous and Robust: A Virtual Roundtable. BioProcess Int. June 2019; https://bioprocessintl.com/manufacturing/continuous-bioprocessing/making-downstream-processing-continuous-and-robust-a-virtual-roundtable.
3 Application Note Version 2: High-Throughput Screening Using Membrane Chromatography. Sartorius: Göttingen, Germany, 2015; https://www.sartorius.com/shop/ww/en/usd/sartobind%C2%AE-q/c/M_Sartobind_Q.
4 Feliciano J, et al. Evaluating High-Throughput Scale-Down Chromatography Platforms for Increased Process Understanding. Wiley-VCH Verlag: Weinheim, Germany, December 2015; https://onlinelibrary.wiley.com/doi/full/10.1002/elsc.201400241.
5 Mittergradnegger D. High-Throughput Process Development Tools for Optimizing a Commercial Process. BioProcess International Summit Europe: Vienna, Austria, April 2019.
6 Horwath B, et al. Raman Spectroscopy Monitoring of Product Quality in CHO Cell Culture. BioProcess International Summit Europe: Amsterdam, The Netherlands, April 2018.
Dr. Margit Holzer is scientific director at Ulysse Consult, 13 Rue de la Fonderie L-1531, Luxembourg; [email protected]. The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any other agency, organization, employer, or company.
You May Also Like