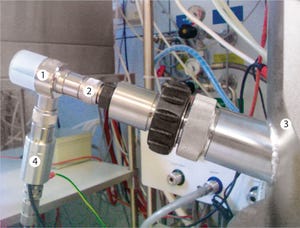
This application note describes the cultivation of Chinese hamster ovary (CHO) suspension cells in the Finesse SmartGlass vessel bioreactor with a maximum working volume of 2.0 L. Using chemically defined minimal media, cell densities of up to 7.44 10
6
cells/mL were achieved. Recombinant secreted embryonic alkaline phosphatase (SEAP) expression was induced by medium exchange and temperature shift. Maximum SEAP activities of up to 63 U/mL were reached. A novel stirred glass bioreactor suitable for cell culture applications at benchtop scale was designed by Finesse Solutions, Inc. With a maximum working volume of 2.0 L, this bioreactor is controlled by the Finesse G3Lab controller.
Introduction
The present study focuses on the cultivation of CHO suspension cells in fed-batch mode using chemically defined minimal culture media and SEAP expression, which is induced by medium exchange and temperature shift. The bioreactor was agitated by a combination of a modified Rushton turbine and a three-bladed segment ...
Successful cell culture on a large scale requires close control of the culture environment in terms of temperature, pH, removal of waste products, and addition of fresh nutrients. To produce high yields of a desired protein, precise control over such parameters at every step in the process is required. Additionally, FDA regulations require various steps to be taken to control bioburden and maintain sterility. These controls and regulations in cell culture environments have led to an increasing move toward single-use systems with the goal of achieving reduced cleaning requirements, high levels of sterility, and zero batch-to-batch contamination (
1
).
Single-Use Solutions
FIGURE 1: DHX cooling efficiences measured by temperature vs. time
Because of contamination concerns, single-use bag systems have gained widespread acceptance and are commonplace in biopharmaceutical manufacturing. More recently, single-use bioreactors for cell culture, single-use tangential-flow filtration (TFF) systems for harvest, and ...
Ladies and gentlemen, welcome aboard ALLpaQ AIR. We have some preflight information about how this bioprocess container optimizes the air freight of media held under temperature-controlled conditions in an environmental chamber.
Before lift-off, please place a single-use bag inside a bioprocess container, fill your media into the bag, and to maintain product temperatures load the bioprocess container inside an Envirotainer RAP e2 container.
Now take count of how many passengers fit inside. One? Two? Three? Four? Owing to the dimensions of standard bioprocess containers, healthcare companies have previously been able to ship only two units per Envirotainer RAP e2 container.
Does this match your calculations? If you could fit only two bioprocess units inside this environmental container, then surely you’d like to fit four? After all, the more media your company ships per flight, the more you save it’s not a case of less is more, but rather more is less.
AIR’s modified footprint doubles the payload, allowing...
Cell culture is widely used for stable expression of monoclonal antibodies with posttranslational modifications. Despite having a stable cell line, a protein product is still in danger of improper modifications from chemical interactions with media. For example, the primary amino groups on a protein are susceptible to reactions with reducing sugar molecules. In the case of a reaction with glucose, this is called
glycation
. The relationship between glucose concentration and glycation is explored here.
Methods:
FIGURE 1: Glucose measurements from three fed-batch runs. Run 1 (square) and run 2 (triangle) were fed once daily. Run 3 (circle) was fed continuously
Chinese hamster ovary (CHO) cells were grown in fed-batch mode in a 2-L glass bioreactor. Offline glucose measurements were obtained using a YSI 7100 system with glucose membrane and two-point calibration. Online measurements were obtained with Biosenz analyzer, with glucose sensor and automated four-point calibration. Glycation of proteins was determ...
Frozen storage is commonly achieved using single-use containers that enable manufacturing process flexibility and long-term product stability as well as minimize logistics challenges. Single-use containers also are available for storing and transporting frozen products although some require a significant investment, and others don’t offer necessary support and protection.
Charter Medical recently developed a single-use frozen storage and transport shipping solution designed to complement single-use Freeze-Pak™ STS (FP-STS) biocontainers, which are engineered specifically for frozen storage applications. The bags and shippers are tested for frozen storage and transport to -80°C. FP-STS single-use biocontainers and shippers have been designed to provide a simple solution while delivering the flexibility and durability required for frozen storage and transport.
The purpose of the study presented here was to test the new Freeze-Pak™ STS single-use secondary storage and shipping containers (Charter Medical, Lt...
Sponsored Content
Through the combined strengths of the New Brunswick™ and DASGIP® product portfolios, Eppendorf now has the product portfolio and expertise to provide an outstanding range of premium equipment and single-use solutions to meet all needs.
A New Scale of Bioprocessing
From the parallel mini bioreactor system DASbox for early stage bioprocess development to the benchtop and parallel bioreactor systems for laboratory scale and the sterilize-in-place solutions for production, Eppendorf offers industry and research users its extensive bioprocess solutions from a single source while meeting the highest quality demands.
Eppendorf Bioprocess Software: Much More Than Just Bioprocess Control
Eppendorf offers BioCommand® and DASGIP Control supervisory control and data acquisition (SCADA) software packages for advanced bioprocess control. next-generation bioprocess management is provided by the comprehensive DASware software suite.
The BioCommand software for New Brunswick line controllers enhances your ability to monit...
With the largest network of harmonized biopharmaceutical good manufacturing practice (GMP) product testing labs worldwide, Eurofins BioPharma Product Testing provides comprehensive laboratory services for the world’s largest pharmaceutical, biopharmaceutical, and medical device companies. We successfully enable companies to advance candidates from development through commercialization while ensuring regulatory compliance, cost effectiveness, and achievement of timelines.
The Most Comprehensive Range of Large- and Small-Molecule Testing Services Available Worldwide
Choose from a wide range of testing services that support all functional areas of biopharmaceutical drug development and manufacturing, including method development, microbiology, process validation, and quality control. Our service offerings are fully comprehensive and include testing of drug substances, final products, intermediates, and starting materials for both small- and large-molecule drug products, including
Global Facilities
Work with ...
Danish-based FeF Chemicals is a preferred specialist supplier of premium quality ingredients for the biopharmaceutical and pharmaceutical industries across two unique product areas: insulin human AF for cell culture media and current good manufacturing practice (CGMP)-manufactured quaternary ammonium compounds (QUATS). And although high quality, innovation, flexibility, and security of supply are among its market-leading features, end-user patients are the motivators. FeF Chemicals supplies insulin human AF (for use in cell culture media) from Novo Nordisk, the largest insulin manufacturer worldwide.
Strong Historic Foundation:
The company was first established in 1949. It was acquired by Novo Nordisk in 1986 and has been part of the pharmaceutical group since then. Novo Nordisk is a renowned global leader with over 90 years of innovation and leadership in diabetes care. The group’s core values are centered on improving the lives of patients. It also strives for excellence, is accountable to customers and...
As major economies in the Asia–Pacific region continue to increase focus on healthcare modernization, new opportunities for both global and local biopharmaceutical companies are being created. Such opportunities are driving demand for local sourcing of raw materials to ensure security of supply.
Modernization of Healthcare Drive Opportunities for Biopharmaceutical Firms
Recent modernization of healthcare by governments has seen China pledge 124 billion to improve its healthcare system to provide insurance to 90% of its residents (
1
). South Korea introduced a biosimilar regulatory pathway consistent with EMA guidelines (
2
) and launched its Bio-Vision 2016 initiative to invest 14.3 billion in 10 years (
3
). India issued its guidelines on biosimilars (
4
) to provide a pathway for approval. And Singapore continues to invest in R&D with its Agency for Science, Technology and Research by signing a five-year strategic agreement with GSK to develop evidence-based formulations targeted specifically for emerg...
To achieve good growth of microorganisms and high yields in protein expression, the following factors in fermentation are important: medium, gas
Fermentors for research and development have to meet very high standards for flexibility so that they can be easily adapted to the requirements of a wide range of microorganisms and process conditions. For that purpose, modular fermentation systems were developed, consisting of single modules such as drive, fermentation vessel, analytical instrumentation, stirrer, foam separator, ventilation, and so on. This allows for individual assembly and upgrades.
metabolism, temperature, pH, and pressure.
The concentration of dissolved oxygen (DO) in medium (proportional to the pO
2
value, which is the partial oxygen pressure that is determined as DO) is an important parameter of gas metabolism. The Hamilton VisiFerm optical DO sensor was tested for four months monitoring this parameter.
The Measurement SiteThe rate of the stirring speed and air flow starts with a defau...
Media and buffer preparation is a key part of the biopharmaceutical manufacturing process. Although this step doesn’t have to be carried out in sterile conditions, improving the powder transfer process makes preparation cleaner, safer, and more efficient while protecting personnel and cutting time and costs.
The Issues of Powder Handling
Powder ingredients for media and buffers have historically been transferred using scoops and weighed and mixed in buckets or open top bags. Many companies are starting to move to single-use systems, but the transfer of dry ingredients still results in fine airborne particles that can cause employee health issues and a slight risk of ignition.
Airborne particles eventually settle onto surfaces, so cleaning between batches must be performed to prevent contamination. This task lengthens the time needed for changeover and increases labor costs.
Process waste also results from using disposable bags that are difficult to fill quickly and accurately. And systems can trap powders...
In the field of research and development into the production of bioactive compounds, there is incredible demand for small-scale cultivation systems that operate in the liter range (1, 2). Single-use bag bioreactors can close the gap between cell and media screening devices (at scales of up to several hundreds of milliliters) and pilot bioreactor systems at scales larger than several hundreds of liters (3). The incubator shaker Multitron Cell with ShakerBag Option, in particular, represents an easy and economical solution for increasing cultivation volumes beyond classical shake-flask scales without the need to purchase a bioreactor system. Besides the general advantages that arise from the single-use design of these bag bioreactors (4), they are also characterized by reduced foam formation and flotation due to the surface aeration (4, 5) that plays an important role in plant cell suspension-based processes.
Identifying engineering parameters such as mixing time, oxygen mass transfer, and power input is im...
Growing adoption of single-use bags for the production of biopharmaceutical drugs raises new challenges, including consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, Sartorius Stedim Biotech’s polymer scientists and biologists have followed a quality by design (QbD) program for the development of a completely new polyethylene film S80 thus achieving consistent performance of the new Flexsafe bags for all bioprocess steps and applications. The partnerships and agreements with our suppliers, the complete understanding and control of our manufacturing process from the resin, and the film extrusion to the final sterile bioprocessing bag are the prerequisites to ensure reliability of our supply chain. This assurance of supply relies on a long-term contract with our film supplier. In addition, the establishment of resin specification instead of general trademarks provides robust change contro...
No longer is the biopharmaceutical industry questioning whether it should use single-use technology. The focus has transitioned to how to best implement single-use systems. Rapid adoption of single-use systems has brought added complexity; which is leading to an increasing application of lean manufacturing principles.
Standardization projects and establishing appropriate standards are current trends that drive toward reducing the complexity of bioprocessing systems, from design and validation to supply chain and manufacturing integration. Although efforts are underway to develop consensus standards for topics such as extractables and leachables and particulates, a number of drug and contract manufacturers are focusing on internal projects to standardize single-use designs across their processes and facilities.
As the market matures, more companies are likely to adopt modular system designs to build the facilities that support multidrug production. Incorporating a catalog of standardized bags, tubing manif...
Advances in cell line engineering and media design have allowed for improvements in monoclonal antibody expression, with cell culture systems exhibiting product titers >10 g/L. These high-density cell cultures pose a great challenge in the use of existing solid liquid separation techniques because of the need to remove larger amounts of biomass and the increase in submicron particles. In addition, the increased level of soluble impurities introduces new bottlenecks for chromatographic processing because of the increased levels of contaminants from cell debris generated during cell culture and harvesting.
FIGURE 1: Particle-size distribution (PSD) of a representative CHO cell culture feed stream (MAb05). Data are shown for unadjusted (pH 7.0), acid treated (pH 4.8), and pDADMAC (0.0375%) treated cell culture feed streams.
The use of flocculation in harvest is a way to overcome these challenges. Controlled flocculation of cell culture suspensions can be used to enhance clarification throughput and downstrea...
As product titers continue to increase in upstream cell culture processes, so too the demand for enhanced capacity in downstream purification steps. MabSelect SuRe LX chromatography medium (resin) is specially developed for monoclonal antibody (MAb) capture from high-titer feeds. To help ensure good process economy, the medium can withstand hundreds of rigorous cleaning cycles with NaOH, with retained dynamic binding capacity (DBC) and MAb yield.
High Dynamic Binding Capacity (DBC)
FIGURE 1: MabSelect SuRe LX exhibits significantly higher DBC compared with MabSelect SuRe medium at 6 min residence time.
Large-scale purification of MAbs usually consists of two or three chromatographic steps, where protein A is the ligand of choice for the initial capture step. MabSelect SuRe LX is based on the same rigid high-flow agarose base matrix and alkali-stabilized ligand as its precursor MabSelect SuRe medium. When compared with MabSelect SuRe, however, MabSelect SuRe LX provides up to 45% higher DBC at 6 min reside...
Purification of therapeutic monoclonal antibodies (MAbs) requires advanced and selective means for separating aggregates from target monomers. Cellufine MAX GS chromatography media are optimized for higher capacity, better separation, and greater selectivity to improve downstream purification of MAbs. JNC Corporation’s new strong cation exchanger, Cellufine MAX GS, has optimized ligand density for improved dynamic binding capacity and selectivity and superior pressure flow characteristics, which are required by MAb purification platforms.
FIGURE 1: Separation of aggregates by stepwise elution.
MAb load = 75 mg/mL resin; column = 5 mm ID 5 cm L, A buffer = 10 mM Na-Acetate pH 4.5 + 50 mM NaCl; B buffer = 10 mM Na-Acetate pH 4.5 + 1 M NaCl; load and wash = 0 % B, first step = 18.5 % B, second step = 100 % B; flow = 0.17 mL/min (load), 0.50 mL/min (elution); sample = HCl treated mouse MAb1 (monomer: 92.6%, dimer = 4.5%, aggregates = 2.9%.
MAb Monomer/Aggregates Separation:
Cellufine MAX GS exhibits exceptio...
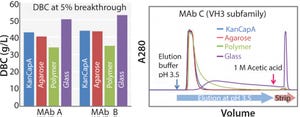
Industrial-scale production of pure monoclonal antibodies (MAbs) customers require an overall highly efficient affinity resin with a high binding capacity, high alkaline stability, good elution profile, low nonspecific binding, and large-scale column operation. Kaneka has developed a novel protein A ligand and immobilized it to highly cross-linked cellulose beads to totally satisfy all these requirements. Here, we demonstrate the performances of the Kaneka KanCapA using MAbs.
FIGURE 1: Dynamic binding capacity (for MAb A and B) and elution profile (for MAb C) of KanCapA compared with commercially available protein A resins; 5% breakthrough is determined at 6 min of residence time (left); 5 mg/mL-resin of IgG (VH3 subfamily) is loaded and elution is performed at pH 3.5. Strip solution is 1 M Acetic acid (right).
FIGURE 2: Pressure/flow rate characteristics of KanCapA packed into various column sizes; mobile phase is water. The pressures generated by packed beds are calculated by subtracting the system pres...
The LEWA EcoPrime® LPLC chromatography platform combines proprietary fluid design and the LEWA Intellidrive® technology to deliver unrivaled batch-to-batch reproducibility and accuracy. The LEWA EcoPrime® features our open architecture automation software and is built on a quality control of the industry’s most vertical supply chain. A single LEWA system has the range of up to three conventional LPLC units while providing a higher level of accuracy. This gives the user great flexibility, more floor space, and plant-friendly integration with simple software customization and updating.
We have combined state-of-the-art functionalities into one unit. It can be a buffer dilution skid or directly connected to a column to be a chromatography system. Tank requirements and labor related to buffer make-up are reduced when the unit directly connects to supplies for purified water and water for injection. This system has been optimized through software analysis of fluid motive, fine-tuned over our 60-year history to...
Strong anion-exchange (Q) chromatography is an industry standard for polishing purification steps of monoclonal antibody (MAb) production. It is a proven technology that removes DNA, viruses, endotoxins, and acidic host-cell proteins (HCPs) from process feed streams in flow-through mode. Advancements in cell culture technology, the emergence of cost-sensitive biosimilars, and the limitations of conventional purification technologies have led to an industry-wide interest in downstream single-use technologies and more flexible biomanufacturing options.
Despite their high binding capacity, traditional resin-based chromatography columns are often oversized because of throughput limitations and are not suitable for flexible biomanufacturing. Conventional membrane adsorbers, meanwhile, offer faster throughput and a certain amount of flexibility, but they cannot provide sufficient process robustness because of the low binding capacity typical of most membranes. These factors impose challenges on the design of pu...
Given the current drive to reduce the cost of manufacturing for biological therapeutics, it is incumbent upon chromatographers and process engineers to streamline the manufacturing process wherever possible. As a result of this need, the demands on downstream unit operations have increased to the point where a single processing step is expected to accomplish a multitude of purification objectives.
Introduction
Anion exchange chromatography is a widely used technique for protein capture or impurity removal. Typically, anion exchange resins with quaternary amine or DEAE ligands are used. However, these more conventional resins have the disadvantage of reduced capacity for proteins in relatively high salt concentrations associated with post-protein A purification monoclonal antibodies (mAbs) or undiluted biological feedstock. In order to use a DEAE or quaternary amine resin at these process stages, the column load material must be diluted to adjust its conductivity to approximately 5 mS/cm.
TOYOPEARL NH2 -75...
Membrane chromatography is an effective alternative to conventional beaded sorbents for the purification of large virus molecules or viral-like particles. Membranes have an open pore structure that allows fast kinetics and direct access to ion-exchange binding sites, with flow rates that are orders of magnitude faster than conventional columns. They provide much higher binding capacities than beaded sorbents for large molecules limited by mass transfer. Pall Mustang ion-exchange membranes are scalable membrane chromatography devices used in various therapeutic, vaccine, and gene therapy products purification schemes. These ready-to-use devices (reusable or single-use) allow users to significantly reduce hardware investments and validation costs typically associated with column chromatography.
A study performed on live influenza (H1N1) showed how structured design of experiments (DoE) and high-throughput screening (HTS) experiments performed on AcroPrep Advance 96-well filter plates with Mustang Q membrane...
This application note describes a case study of the correct sizing for a filter involved in an automated final bulk-fill system for a biopharmaceutical product. It demonstrates how customer batch-processing requirements can be attained with a robust experimental design.
Scale-Up Methodology
Figure 1 shows a process for successful scale-up. Once the desired process has been defined, filter performance can be determined by either constant flow or constant pressure testing on disc formats.
FIGURE 1: A process for successful scale-up
The data can be scaled-up easily to determine the required system size for the target process because capacity is directly proportional to filter area. Pilot-scale or full engineering trials can be used to confirm initial findings because experimental variability can be introduced through differing filter formats, drug product batches, or process equipment.
Sizing should be performed using a scale model of the target process. Ideally, processing time should remain constant, and t...
The increasing adoption of single-use technology in biopharmaceutical, vaccine, and cell therapy production is one indication that such technology has moved far beyond its novelty stage. Arguably, this is the preferred technology of newly developed processes. To support the continued growth of single-use systems in the biopharmaceutical industry, the ability to measure critical process parameters with single-use sensors is required. Several key single-use sensing technologies are described, including pressure, temperature, UV absorbance, and conductivity. The technology behind each is explored as well as examples of the sensors in practical applications.
Pressure Sensors:
Single-use sensors for pressure measurement have already become widely accepted and adopted. This is partly attributed to the fact that pressure is the most critical measurement related to process performance (as well as safety) for many different bioprocesses. Products such as PendoTECH single-use pressure sensors are used to measure p...
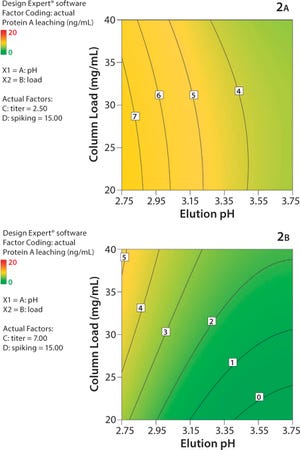
Protein A chromatography has become a widely used platform in monoclonal antibody (MAb) purification. We describe the impact of higher MAb loadings on aggregation and ligand leaching during protein A chromatography, as well as the benefits of high-capacity Protein A resins for high-titer feedstocks. Today, high MAb titers achieved through advanced upstream processing have become a challenge for downstream processing, especially with regards to the expensive protein A capturing step. TOYOPEARL AF-rProtein A HC-650F provides superior MAb capacity and beneficial MAb uptake behavior and can thereby increase capturing productivity.
MAb Adsorption:
Because dynamic capacity depends on flow and feed concentration, it was tested at various residence times (RT) and MAb titers. Figure 1 shows the breakthrough curve for TOYOPEARL AF-rProtein A HC-650F at a feed concentration of 10 g mAb/L. The resin shows complete MAb adsorption until breakthrough occurs even for short RT of 1 min. The capacities of more than 100 mg...
Biosimilars are set to dramatically improve how millions of people access expensive, complex, life-saving treatments by providing equally effective alternatives to innovator products while lessening the cost burden (>30%). Therapeutic Proteins International, LLC (TPI) is a fully integrated manufacturer of biosimilar recombinant protein products that has reinvented conventional approaches to biologic drug manufacturing.
Powerful Science Shouldn’t Be Complicated
TPI has reinvented the process used to manufacture biological drugs making it simpler by leveraging superior technology and rigorous scientific methods to significantly reduce the number of steps involved. At the core of TPI’s patented manufacturing platform are disposable bioreactors and a totally single-use system. Those proprietary systems allow for quick scale up and simultaneous development, which reduce the timeline and costs associated with bringing products to market. In addition, TPI performs analytics at each step to optimize performance a...
Sponsored Content
Major Markets
AbbVie Contract Manufacturing serves domestic and global companies seeking to outsource biologics, potent capabilities, drug products, fermentation, prefilled syringe manufacturing, and HME, as well as businesses looking to procure APIs. Outsourcing clients range from mid-size to large pharmaceutical and biotech companies.
Services Offered
Biologics:
AbbVie is a leading biopharmaceutical developer and manufacturer, with a scientific team that offers expertise in process optimization, analytical characterization, and current good manufacturing practice (CGMP) manufacturing to support full development and commercialization of biologics.
Potent Capabilities:
AbbVie Contract Manufacturing offers a full range of potent capabilities for drug product and highly potent active pharmaceutical ingredients (HPAPIs), covering development phases to commercial equipment capable of handling potent classification of 3.
Drug Products:
AbbVie Contract Manufacturing offers a full range of drug product manufa...
Aeras is a nonprofit biotech focused on the development of TB vaccines. Together with our global research and development partners, we have played an integral role in developing approximately half of all TB vaccine candidates in clinical development around the world.
TB is one of the most deadly infectious diseases in the world. It spreads through the air like the common cold. A TB patient with active disease can infect up to 15 people simply by coughing, sneezing, or talking. The emergence and spread of drug-resistant forms of TB are complicating multinational efforts to control the global epidemic.
Because TB is more complex and more difficult than ever to treat, it presents a worldwide health threat. So attacking this challenge requires developing expertise and experience in multiple biologics technology platforms. Aeras approach includes building integrated, state-of-the-art, end-to-end development and manufacturing capabilities for translational development support all under one roof.
Aeras is levera...
Avid Bioservices is a contract manufacturing organization (CMO) specializing in mammalian cell culture process development and current good manufacturing practice (CGMP) production of clinical- and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to your success, our team is constantly striving to build partnerships that extend well beyond the delivery of product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements.
Established CMO with Proven Capabilities
Excellence in Process Science
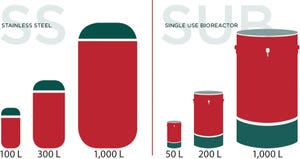
FIGURE 1: Bioreactor capacity
Avid’s process sciences group is focused on the development of process and analytical methods needed to deliver active products for transfer to CGMP manufacturing. Our experience with CHO-based IgG production, as well as knowledge in the areas of recombinant protein and enzyme process development, provide the expertise you need to advance your programs to the next phase. Expertise in...
Boehringer Ingelheim BioXcellence™ is your dedicated biopharmaceutical contract manufacturer. As a leading biopharmaceutical contract manufacturer with more than 35 years of experience, it has brought 22 biopharmaceutical products to market. Boehringer Ingelheim BioXcellence™ offers tailor-made contract development and manufacturing services to the biopharmaceutical industry, providing the entire production technology chain from DNA to fill and finish under one roof at its facilities in Biberach (Germany), Vienna (Austria), Fremont (USA), and Shanghai (China). Boehringer Ingelheim BioXcellence™ can secure product supply throughout the entire product life cycle transferring customer projects at any stage, delivering to almost any scale. Boehringer Ingelheim BioXcellence™ makes outsourcing easy.
Boehringer Ingelheim BioXcellence™ Services
At our cell culture sites in Biberach (Germany) and Fremont (USA), we have a total capacity of more than 200,000 L, with flexible scales for clinical and commercial supply...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.