Purifying Common Light-Chain Bispecific AntibodiesPurifying Common Light-Chain Bispecific Antibodies
A bispecific antibody can bind two different antigens. Immunoglobulin G (IgG) type antibodies have two binding sites with different variable regions. An IgG variable region is made up of a variable light-chain sequence (VL) and a variable heavy-chain sequence (VH). The light chains (LCs) of common LC antibodies are identical for both variable regions, leaving the heavy chain (HC) for generating different specificities. Thus, recombinant host cells for production of common LC bispecific antibodies carry genes for both HCs, with the different specificities (A and B), along with one LC gene. A, B, and the light chains are expressed independently in those host cells, which then assemble them into three IgG types — AA, AB, and BB — for secretion into a culture environment. By purely random assembly, the three types should be produced in a ratio of 1:2:1 (AA, AB, and BB; AB and BA are equivalent). Purity of AB with respect to AA and BB thus would be only 50%.
PRODUCT FOCUS: BISPECIFIC ANTIBODIES
PROCESS FOCUS: DOWNSTREAM PROCESSING
WHO SHOULD READ: PROCESS AND PRODUCT DEVELOPMENT
KEYWORDS: AFFINITY CAPTURE, ION-EXCHANGE POLISHING, BIND-ELUTE, FLOW-THROUGH, ANALYTICAL CHROMATOGRAPHY, PROCESS CHROMATOGRAPHY
LEVEL: ADVANCED
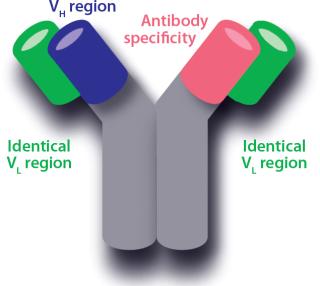
Common light-chain antibody ()
Genetic engineering methods can modify heavy-chain Fc regions such that A and B are assembled preferably, thus reducing formation of AA and BB. In charge engineering, for instance, negative amino-acid residues are engineered into one HC CH3 region whereas positive residues are engineered into the complementary region of the other heavy chain. That results in bispecific IgG production containing no more than trace amounts of the homodimeric parents (1). In general, preserving the IgG backbone should minimize immunogenicity and provide pharmacokinetics similar to those of natural monoclonal antibodies (MAbs) (2). Although such CH3 engineering approaches can generate highly pure, bispecific IgG, there may be a need in certain applications for bispecific IgG containing wild-type unmodified Fc Regions. Choices made for downstream processing thus would play a decisive role for purifying those bispecific antibody species.
Twin-Column MCSGP Chromatography
Multicolumn countercurrent solvent gradient purification (MCSGP) is a chromatographic process that allows for product isolation with high yield and purity in difficult separation situations. In many cases (e.g., bispecific antibody isolation), the difficulty is caused by product-related impurities that elute closely to the product of interest. Often in preparative batch chromatography, the overlap remains even after reasonable elution-condition optimization, allowing for isolation of only a small product fraction at sufficient purity.
Increasing the product fraction leads to inacceptably reduced purity. Therefore, a large amount of product contained in the impure side-fractions flanking the product peak gets discarded due to insufficient purity. This situation is typically worse when a chromatography step operates at high load because the product–impurity overlap is enhanced by mass-transfer effects. Thus, in addition to the trade-off between yield and purity, there is also a trade-off between productivity and purity.
The twin-column MCSGP process alleviates those trade-offs through internal recycling of the impure-side fractions. Those fractions containing overlapping product and impurities are washed from one column into the other, where the product is readsorbed and conserved. Figure 1 outlines this four-phase process.
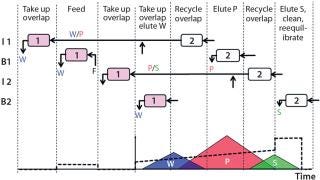
Figure 1: ()
Four Phases: In phase 1, the columns are interconnected, and the chromatographic fraction containing the overlapping region of weakly adsorbing impurities and product elutes from the upstream into the downstream column. In between those two columns, the stream is diluted inline to ensure readsorption of eluted product on the downstream column.
In phase 2, the columns are disconnected, and pure product elutes from the upstream column. The overlapping region of product and strongly adsorbing impurity remains in the upstream column. In parallel, feed solution is loaded onto the downstream column.
In phase 3, the columns are again interconnected, and the chromatographic fraction containing the overlapping region is washed from the upstream into the downstream column. Again, product adsorption in the downstream column is ensured by inline dilution of the stream exiting the upstream column.
In phase 4, the columns are disconnected again, and the strongly adsorbing impurities are washed out of the upstream column. That column is stripped, cleaned, and equilibrated. In parallel, weakly adsorbing impurities elute from the downstream column as elution is initiated.
After that last phase, the columns switch position so that the (equilibrated) upstream column becomes the downstream column and vice versa. Phases 1–4 repeat with the columns in this reverse order. On finishing phase 4 and another column-position switch, the columns have returned to their original positions, so one cycle of the twin-column process is complete. It continues to run over multiple cycles (Figure 1).
MCSGP elution runs in analogy to the elution of a single-column batch process, so operating parameters (pump flow rates and gradient concentrations) can be derived directly from batch-process operating conditions once distribution of product and impurities is known. That distribution (indicated schematically in Figure 1) is typically derived either from the UV signal or from offline analyses of fractions collected during batch chromatographic elution. Knowing the distribution allows for determination of different chromatographic sections: a section with pure W, one in which W and P overlap, a section of pure P, a section where P and S overlap, and a section of pure S. In this case, W = AA, P = AB, and S = BB. The elution volumes and gradients to be run through the upstream column are defined for each process phase by elution volumes between those section borders. Gradients are defined by concentration values at the section borders.
Materials and Methods
Merus supplied clarified PER.C6 cell culture harvest containing a common light-chain bispecific antibody mixture. The parental antibodies had isoelectric points at pH 7.3 (AA) and pH 8.4 (BB). The target bispecific antibody AB was 35% of the clarified harvest, accord
ing to analytical cation-exchange (CIEX) gradient chromatography using a Tosoh SP STAT 7-µm, 4.6-mm × 100-mm column. Overall antibody concentration was 1.7 g/L of AA, AB, and BB together. Before our purification work, the harvest was filtered using Sartobran 300 cartridges from Sartorius Stedim Biotech.
Purity Specifications: Native mass spectrometric (MS) analyses on process intermediates and final, purified AB bispecific antibody material was performed as described in the literature (3). We set our specifications as follows:
<0.5% AA and BB main isoform content based on analytical CIEX chromatography as above
<1.0% aggregates based on analytical size-exclusion (SE) chromatography (Superdex 200, 10/300 GL from GE Healthcare)
<30 ppm host-cell protein (HCP) content according to a Cygnus Technologies PER.C6 enzyme-linked immunosorbent assay (ELISA)
<10 ppm DNA according to a QuantIT chromogenic assay from Invitrogen (Life Technologies).
Chromatography: We chose Tosoh rPA for our protein A capture step based on HCP clearance data. A targeted screening identified suitable CIEX stationary phases. We selected preparative stationary phases for this downstream process based on the peak shapes of gradient-elution chromatograms obtained from analytical injections. We adapted those gradients (without changing their slope) for similar peak retention times. Under these conditions, Poros 50HS displayed the shallowest peaks — indicating the best mass-transfer properties — and was therefore our choice for the first polishing step (CIEX). For the second polishing step, we chose Toyopearl SuperQ 650 M (also from Tosoh Biosciences) because it allowed for high yields from directly loaded CIEX eluate.
Whenever possible, we used generic buffers and procedures throughout this process (Table 1). We held the protein A eluate at pH 3.2 for 60 min before neutralizing it to pH 5.5 using 1M Tris at pH 8.5. Then we diluted the eluate 1:2 (one part eluate, one part deionized water) before loading it onto the CIEX columns. But we did not treat the CIEX eluate before loading it onto the anion exchange (AIEX) column.
Table 1: Overview of stationary phases and buffers
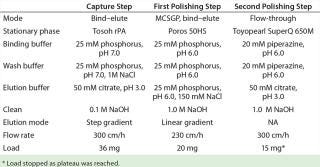
Table 1: Overview of stationary phases and buffers ()
Affinity Capture Results
Protein A chromatography efficiently captured antibodies AA, AB, and BB from clarified harvest with a yield >95% of total IgG. Figure 2 is a representative chromatogram of a capture run.
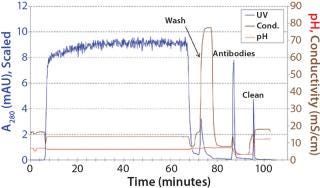
Figure 2: ()
Although protein A cannot distinguish between the bispecific antibody AB and parental antibodies AA and BB, it is an indispensable part of all IgG antibody platform processes because of its excellent impurity clearance. In our case, HCP content was reduced by two orders of magnitude (2 logs) from 46,000 ppm to 200 ppm. Figure 3 shows an analytical CIEX chromatogram of product obtained from the protein A capture step. Note that only a few impurities responsive at 280 nm are present (the oval).
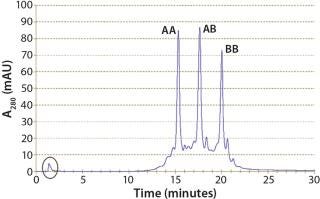
Figure 3: ()
With low-pH elution, furthermore, a low-pH virus inactivation step is basically integrated into the protein A capture. Parameters to be optimized are elution pH and duration of the low-pH hold. In our case, we confirmed with SE high-performance liquid chromatography SE-HPLC that no additional aggregates formed during the hold step at pH 3.2 over 60 min (Figure 3).
CIEX Polishing Results
In our platform, the protein A step is succeeded by a CIEX step in bind–elute mode for removing the parental antibody species AA and BB. Using single-column chromatography, we found gradient and load conditions that allowed for isolation of the bispecific antibody species AB with <0.5% AA and BB main isoform remaining. The load was 20 mg of total antibody per milligram of stationary phase, with a linear-gradient elution. We optimized chromatography conditions no further than that, but an increase in gradient duration might increase the yield — at a cost of throughput (Figure 4).
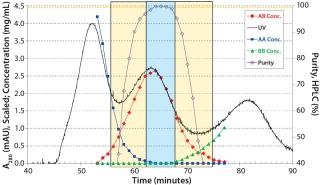
Figure 4: ()
We used a single-column batch chromatogram as a basis for determining our MCSGP operating parameters: two flow rates per phase (1, 2, 3, and 4) and gradient concentrations in the upstream column (Figure 1). Once the regions for internal recycling of impure-side fractions (overlapping parts AA–AB and AB–BB, respectively) are selected based on offline analysis results (left and right rectangles in Figure 4), pump flow rates can be calculated based on the elution volumes of the single-column batch flow rate at the section borders (and based on the section width). Gradient concentrations correspond to the those at the section borders. The feed flow rate is set according to the amount of product that is expected to elute from the process during the cyclic steady state (center rectangle in Figure 4). Inline dilution during the internal recycling steps and reequilibration uses buffer A, and 1.0 M NaOH is used for cleaning.
We outlined our operating parameter determination procedure previously for a six-column MCSGP process (4, 5), and we are detailing the procedure for a two-column process in a future article. Using operating parameters derived from the single-column batch chromatogram, we operated our MCSGP process to a cyclic steady state. At that point, product purity, yield, and concentration remain constant and the mass balance closes. In the case presented here, purity was within specification from the very first switch-on.
Figure 5 (LEFT) shows an overlay of analytical chromatograms for CIEX feed material with product obtained from a representative MCSGP run. The MCSGP process completely removed the main isoforms of parental antibodies AA and BB were removed completely by the MCSGP process.
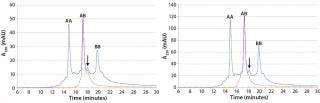
Figure 5: ()
In those analytical CIEX chromatograms, the basic AB isoform is present in the MCSGP product pool. Although that isoform can be attributed to AB, we ran MCSGP a second time to demonstrate its capability to remove the basic isoforms of AB along with parental antibodies AA and BB. Figure 5 (RIGHT) shows an overlay of product obtained from that second run with the CIEX feed material. Suitability of MCSGP for separating antibody isoforms has been shown earlier (6, 7).
We also analyzed the AB product from MCSGP using SE-HPLC to determine aggregate content. Figure 6 shows an overlay of the SEC chromatograms of CIEX starting material with product from MCSGP. The figure also zooms in on the aggregate region of the chromatograms, showing excellent aggregate clearance achieved.
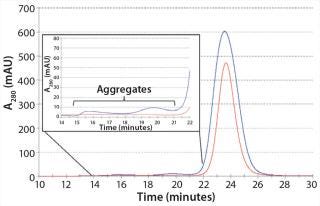
Figure 6: ()
Comparing Single-Column Batch and MCSGP Chromatography: We compared batch and MCSGP processes in terms of yield for a given productivity and <0.5% main isoform content (according to CIEX analytics). The AB yield from the batch process was just 37% because only a narrow pool fraction could be chosen under the given purity constraint. Widening the elution window (indicated by the center rectangle in Figure 4) would increase the AB pool yield but reduce its purity. Plotting purity over yield for different AB pooling ranges illustrates the yield–purity trade-off inherent to difficult separations in which product and impurities overlap (Figure 7). In this case, only the points located above the horizontal line represent product pools that fulfill the purity specification (<0.5% AA and BB main isoform content). We ran our MCSGP over multiple cycles and operated at a steady-state yield of 87% with <0.5% AA and BB main isoform content. This operating point is a single data point (diamond) in the upper-right corner of Figure 7. Productivity was comparable for single-column and MCSGP chromatography processes (1.6 g/L/h ± 10%).
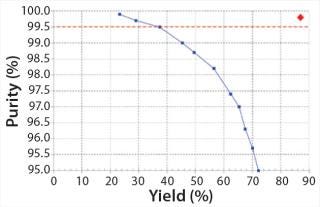
Figure 7: ()
Note that productivity is calculated with respect to the target AB (which makes up just 35% of the load material’s total antibody content) and that it is not optimized. As stated above, simultaneous achievement of high yield and purity from the MCSGP process comes from internal recycling of the impure-side fractions (overlapping parts in Figure 4) (Figure 7).
AIEX Polishing Results
The CIEX eluate was further polished with an AIEX step in flow-through mode. We loaded the CIEX eluate onto an AIEX column without further adjustment. As soon as UV, pH, and conductivity signals reached a plateau, we took a sample of the flow-through and analyzed it offline. Figure 8 is a chromatogram of the AIEX flow-through step. We confirmed with analytical CIEX chromatography that the isoform pattern of the AB antibody was not changed by this second polishing step.
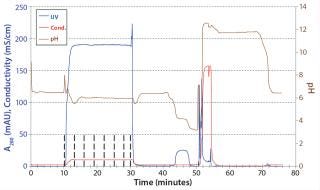
Figure 8: ()
The main isoforms of AA and BB were removed to final amounts of <0.5% during this downstream process, as determined using analytical CIEX. However, native MS data for the final products showed that ∼20% of BB isoforms and ∼10% of AA isoforms were present in the final product. Such a large content of non-AB isoforms comes from the fact that some AA and BB isoforms have adsorptive properties that are nearly identical to those of the main AB isoform and thus coelute with it in both preparative and analytical CIEX chromatography. In addition, AA and BB isoform concentrations are quite high in the cell culture harvest (Figure 8).
We also analyzed our final product for HCP, DNA, and aggregate contents. Table 2 summarizes those purity results.
Table 2: Analytical results for clarified harvest and product analyzed after protein A, MCSGP CIEX, and AIEX chromatography; ranges are given when products were analyzed more than once.
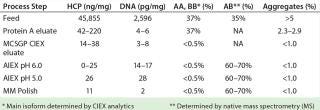
Table 2: Analytical results for clarified harvest and product analyzed after protein A, MCSGP CIEX, and AIEX chromatography; ranges are given when products were analyzed more than once. ()
A New Kind of Platform for a New Type of Product
We developed a production platform process for bispecific IgG antibody purification. It includes the following chromatographic steps in sequence: protein A affinity chromatography (in bind–elute mode), CIEX chromatography (MCSGP process in bind–elute mode), and anion-exchange chromatography (in flow-through mode). In this case, the final product contained <30 ppm HCP (determined using a generic ELISA), <1% aggregates, and <0.5% AA and BB main isoforms (from a feed content of 37%).
Running the CIEX chromatography process in MCSGP mode enabled isolation of a target bispecific antibody AB at an 87% step yield. By contrast, running the CIEX step in batch mode for comparison delivered AB only at a 37% yield. Overall yield (of the chromatography steps) was 78% for a downstream process including MCSGP CIEX and 34% for the same process including batch CIEX. Table 3 overviews the process yields for both downstream processes.
Table 3: Analytical results for clarified harvest and product analyzed after protein A, MCSGP CIEX, and AIEX chromatography
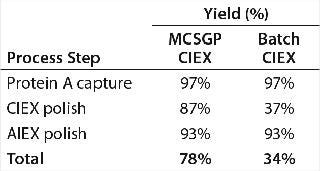
Table 3: Analytical results for clarified harvest and product analyzed after protein A, MCSGP CIEX, and AIEX chromatography ()
MCSGP also can be used to remove isoforms of the target bispecific antibody AB, which represents an even larger separation challenge than that of separating AB from the main isoforms of the homodimeric antibodies AA and BB. MCSGP purification capabilities are limited, however, if certain isoforms of AA and BB coelute with the AB main isoform due to identical adsorptive properties. In such cases, an orthogonal chromatographic step would be needed using a different adsorption principle (e.g., multimodal chromatography or hydrophobic-interaction chromatography) to separate those coeluting species.
It can be concluded that our downstream platform process can be used to separate bispecific antibodies AB from mixtures containing even close-eluting parental antibodies AA and BB, provided that their charge heterogeneity is limited and that single isoforms of one species do not overlap with the main isoform of the other species in CIEX chromatography. This process — including the CIEX-MCSGP step — is based on commercially available materials with particle sizes of 45–65 µm, which allows for high throughputs and good scalability.
About the Author
Author Details
Corresponding author Thomas Müller-Späth is chief scientific officer, Nicole Ulmer is a scientist, Lars Aumann is chief technical officer, Guido Ströhlein is chief executive officer, and Michael Bavand is chief business officer at ChromaCon AG, Technoparkstrasse 1, 8005 Zurich, Switzerland; 41-44-445-2011; [email protected]; www.chromacon.ch. Linda J.A. Hendriks is a scientist, John de Kruif is chief scientific officer, Mark Throsby is chief operating officer, and Alexander B.H. Bakker is chief development officer at Merus BV, Padualaan 8, 3584 CH Utrecht, The Netherlands.
REFERENCES
1.) Gunasekaran, K. 2010. Enhancing Antibody Fc Heterodimer Formation Through Electrostatic Steering Effects: Applications to Bispecific Molecules and Monovalent IgG. J. Biol. Chem. 285:19637-19646.
2.) Cain, C. 2011. Crossing Over to Bispecificity. SciBX 4:783.
3.) Rosati, S. 2012. Qualitative and Semiquantitative Analysis of Composite Mixtures of Antibodies By Native Mass Spectrometry. Analyt. Chem. 84:7227-7232.
4.) Aumann, L, and M Morbidelli.
5.) Aumann, L, and M. Morbidelli. 2007. A Continuous Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) Process. Biotechnol. Bioeng. 98:1043-1055.
6.) Müller-Späth, T. 2008. Chromatographic Separation of Three Monoclonal Antibody Variants Using Multicolumn Countercurrent Solvent Gradient Purification (MCSGP). Biotechnol. Bioeng. 100:1166-1177.
7.) Müller-Späth, T. 2010. Increasing the Activity of Monoclonal Antibody Therapeutics By Continuous Chromatography (MCSGP). Biotechnol. Bioeng. 107:652-662.
You May Also Like






