Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 2: Clinical Efficacy, Reimbursement, and Needle-to-Needle LogisticsPlanning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 2: Clinical Efficacy, Reimbursement, and Needle-to-Needle Logistics
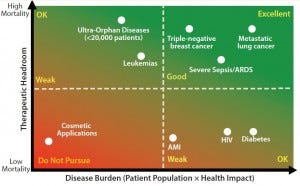
Figure 1: Assessing your therapies’ reimbursement potential
Cell therapy is an emerging pillar in healthcare with the potential to provide curative solutions to a wide range of indications. The biological complexities through which cell technologies exert their clinical impact (especially those used in immunotherapies for cancer) provide opportunities for novel modes of immune regulation, cell targeting, and payload delivery. Cells also can serve as vehicles for genetic content, which the gene therapy industry is now investigating.
Since early 2004, Invetech has worked with organizations dedicated to cell therapy and regenerative medicine to help them develop and implement commercial-scale manufacturing for a broad range of therapies. In part 1 of this two-part article (1), we introduced the elements for building a solid foundation for game-changing cell-therapy product development. We call them the “five pillars of success”:
manufacturability
cost of goods
efficacy
reimbursement
needle-to-needle logistics.
Part 1 focused on the first two pillars of success (manufacturability and cost of goods) (1). Herein we discuss efficacy, reimbursement, and needle-to-needle logistics as they relate to the requirements for planning commercial-scale manufacture of products related to cell therapies and regenerative medicines. Topics include trade-offs and decisions that must be made to prevent the “valley of death,” thereby enabling a successful progression to commercialization.
Efficacy
Efficacy is the primary (and in some cases, sole) focus of most young companies. Because many biotechnology start-ups are launched on the merits of scientific technology that is developed in academic institutions and then patented, it is not surprising that their initial priority is to produce positive experimental results (a learned academic behavior).
In a biotechnology start-up, investigators must demonstrate surmounting myriad hurdles of evolving science from concept to proof in cells. They must prove the possibilities in small-animal models, then in larger animal models; win the multitude of grants necessary to complete their work; convince technology transfer-office agents to fast-track their technologies; run experimental programs; and finally, patent and publish their work. This business model transfers directly from a deeply embedded desire to meet clinical trial milestones. However, for biotechnology start-ups to be fiscally solvent in the long run, it is critical to also maintain a broader business focus. Although a company has nothing without clinical efficacy, achieving such without other successful business practices in place is a fast road to failure.
The emphasis on efficacy is understandable, but other fundamental factors for product success cannot be postponed for long. Once phase 1 trials have proven a therapy to be safe (and, for reimbursement, even before then), concrete plans should be developed for other aspects of a successful business. The following must be addressed when starting a planning process:
How will we manufacture consistently good product?
How will we manufacture an affordable product?
How will we receive starting materials, manage manufacturing at industrial scale, and deliver the product to our patients?
Is there enough headroom between standard of care and reimbursement for us to exist as a business?
Reimbursement
Although cell therapies are revolutionary and have instilled much excitement in the biotechnology industry, they can be expensive to produce and deliver. And they are arriving at a time when healthcare payers are weighing up the benefit- versus-cost equation. For cell therapies to be successfully adopted into healthcare systems, developers must be critical of cost-effectiveness from the earliest stages of development. Although reimbusement is the last milestone in the long path to revenue generation, it is perhaps the first consideration to make when deciding the viability of a cell therapy product for a particular indication.
How do we stratify indications to identify those with the greatest prospects for reimbursement? Perhaps the simplest approach is to analyze mortality data. Mortality in a patient population represents the shortcomings of an existing standard of care. Where mortality is high, standard of care is low, and willingness to pay by reimbursement providers is high. Conversely, where mortality is low, standard of care is high, and payers exhibit considerably less willingness to pay (Figure 1).
To illustrate this point, consider two hypothetical therapies: a mesenchymal stem cell line being developed for treatment of acute respiratory distress syndrome (ARDS) and a bone-marrow progenitor cell line being developed for treatment of acute myocardial infarction (AMI). The mortality resulting from ARDS is 40%, whereas that of a single AMI is only a few percent. The margin in which a cell therapy developer can show an improvement over standard of care in ARDS is quite large, whereas there is virtually no room for improvement in the way AMI is currently managed by physicians. The amount that a reimbursement provider should be willing to pay for either of these technologies is quantifiable and can be calculated readily.
An equally important factor for maximizing payout from reimbursement providers is average age at incidence. For example, the fewer years remaining in life, the lower the willingness to pay. To view this from another perspective, the fewer years remaining in life reduces the opportunity for a therapy to deliver a health impact to a patient. Would it be a rational decision to develop a macrophage cell therapy to treat pneumonia in elderly patients? No. The average age of incidence of pneumonia in this patient group is about 75, whereas the average life expectancy in the Western world is about 80. Even for diseases with high mortality rates, the willingness to pay for such disease therapies would be nominal because cost-effectiveness calculations would incorporate five years of remaining life.
Now flip that scenario on its head. Would you develop a macrophage cell therapy to treat pneumonia in the pediatric population? Many people would say that this is an idea with commercial potential. Necrotizing pneumonia associated with methicillin-resistant Staphylococcus aureus (MRSA) has an average age at incidence of 14 and mortality of 50%, which is an optimal setting for reimbursement. However, the incidence of this indication may be prohibitively low to warrant commercial development. Other subsets of pediatric pneumonia patients provide more attractive markets. For example, premature babies often experience difficulties with their lungs and frequently get infections if intubation is required.
When deciding on an acceptable cost-of-goods (CoG) for a potential cell therapy, it is best to identify the target indication and work backward. Once an indication is selected, one can then calculate a willingness-to-pay value using mortality or quality-of-life data (willingness to pay generally ranges between $50,000 and $100,000 per quality-adjusted life year, depending on jurisdiction). The best-case scenario is that your cost of goods will be about 25% of that value. But generally it will need to be less than that value to account for costs associated with labor, manufacturing, logistics, quality control assays, and release testing. CoG reduction and process development and optimization are critical components of a cell therapy development plan.
Mortality data and age of incidence are high-level approaches that allow for rapid assessment of reimbursement potential. Given the high cost of cell therapies, they should be an integral part of indication selection. Studying the reimbursement landscape to identify trends in payers’ treatment of various products also is important. For example, a company developing nanoparticle therapies would need to know that Medicare lumps cellular and acellular products into the same reimbursement category for wound healing, creating significant challenges for cell therapy technologies.
A company can evaluate its therapeutic candidate’s reimbursement potential (and subsequent long-term viability of the company itself) by
understanding the mortality and standard of care for the therapy
calculating willingness to pay in quality-adjusted life years
calculating CoG to be 25% or less of willingness to pay.
Needle-to-Needle Logistics
Needle-to-needle logistics is an industry term that refers to all of the little details that need to be performed well to make a business function.
What must be addressed for a cell therapy company to successfully transition to profitable operation at commercial scale? The issue is not only a matter of overcoming logistical challenges of distributing, delivering, and administering outgoing product, nor of resolving similar challenges of inventory management and industrial- scale manufacturing. It is also managing the challenges of shipping, receiving, and tracking incoming items (e.g., cells, reagents, and disposables). Those tasks are all significant and time critical.
Cell therapy manufacturers seeking to distribute and administer market- winning products must develop and refine all their logistical processes to be robust, repeatable, and error-proof while allowing and recording traceability to guarantee chain of custody. These processes must cover
packaging and storage (both temperature-controlled and ambient)
management of inventory (stock control and temperature-monitoring systems, both in stores and during distribution)
clinical-site capabilities that affect product and administration quality
timeliness to ensure that patient- specific therapies are delivered to the correct patients.
Such topics worthy of entire articles in themselves. For brevity, the boxes here include listed sets of questions for a given company to answer as a means of successful organizational planning and process implementation.
Five Pillars of Success
To both summarize the box questions and recapitulate the main take-away points from part 1, below is a summary of best practices from our “Five Pillars of Success” that will set you on a path to becoming a thriving cell therapy business.
Manufacturability: Close the process to enable manufacturing in a large, open-plan, common manufacturing area.
Cost of Goods: Optimize the manufacturing process to minimize costs while still consistently producing a product that meets identified critical quality attributes (CQAs).
Efficacy: Refine that process for industrialization by removing skilled labor and art.
Reimbursement: Analyze the window of reimbursement for your therapy to determine whether you have a prospective business.
Needle-to-Needle Logistics: Map out early how your logistics might all fit together, and identify the issues still in need of solutions.
Incoming Logistics |
|---|
Source Materials: Do they need to be temperature controlled? Will they be fresh or frozen? How long will they take to ship, and what abnormal temperature events might take place during transportation? |
Reagents: What format (fresh, frozen, or lyophilized) will your reagents come in? What is the right packaging for appropriate batch size? Will you need a sterile connection to closed processing disposables? Consider also implementing stock control and “Use by” dates. |
Sterile Disposables: What packaging will be used? What kind of sterile connections are needed for closed processing? Stock control and storage life should also be considered here. |
Data: How will patient IDs be created, stored, and tracked? How will batch and lot numbers be maintained? Where and how will receipt, manufactured, and expiration dates be recorded? |
Outgoing Logistics |
|---|
Product: Will the product be frozen or fresh? How will you manage and monitor temperature, both in transit and in storage? |
Quality Control: How and where will testing occur (in-house or external)? Where will retained samples be stored and for how long? |
Final-Product Vessel: Will the vessel come from a custom or an original equipment manufacturer (OEM)? How will you match the selected dose container to the appropriate final shipping container? Make sure to consider the ease of access and administration of dose when determining the vessel. |
Waste: Will you incinerate on-site, or hire an external contractor for biological waste disposal? |
Data: What will be the chain of custody for data? How will you retain shipment records, including temperature history and proof of waste destruction? |
Clinical-Site Logistics |
|---|
Receiving and Storage: Does the clinical site have the facilities and processes to receive and appropriately store the product? |
Dose Preparation: Can the clinical site thaw the product and conduct whatever preparation is needed (preferably minimal) before administration? |
Administration: Are staff adequately trained to administer the therapy? Does its administration differ from a standard method? Additional training and complexity will affect uptake and likelihood of compliance with specified methods. |
Data: What will be the chain of custody? How will you handle the record of administration and clinician training records? |
Inventory, Work-in-Progress Manufacturing Logistics |
|---|
Patient Material: How will you ensure that cold-chain supply has been maintained within set boundaries? |
Reagents: How will you manage inventory? How will you prepare batches and/or mix them before use? How will you manage first-in/first-out (FIFO) and expiration control? |
Disposables: Consider inventory control as well as FIFO and expiration control. |
Scheduling: What software and processes will you use for production scheduling, equipment availability/use, and operator availability/use? |
Freeze–Thaw Decision: Will you use a freezing step to simplify planning? How will you manage temperature control and history of products at key processing steps? |
Data: How will you manage manufacturing execution system (MES) selection and integration? How will you manage batch records, traceability, and chain of custody (including matching patient IDs, patient material, reagent and disposable batch and lot numbers, received dates, manufactured dates, expiration dates, and processing operator IDs)? How and where will you store equipment calibration history as well as operator history and training records? |
Reference
1 Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 1, Achieving Manufacturability and Managing Cost of Goods. BioProcess Int. 13(4) 2015: S6–S8, S19.
Corresponding author Richard Grant is global vice president of cell therapy at Invetech, 495 Blackburn Road, Mt. Waverley, Melbourne, VIC 3149, Australia; Richard. [email protected]. Contributor Mark Curtis is an analyst at CCRM, 100 College Street, Suite 110, Toronto, ON M5G 1L5, CANADA; [email protected]. Meredith Brown is director of business development at Invetech, 9980 Huennekens Street, San Diego, CA 92121; [email protected].
You May Also Like





