Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck ManufacturingAutomation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
 Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1). Then in March 2015, the European Medicines Agency’s (EMA’s) Pediatric Committee agreed on a pediatric investigation plan for tisagenlecleucel-T (2).
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1). Then in March 2015, the European Medicines Agency’s (EMA’s) Pediatric Committee agreed on a pediatric investigation plan for tisagenlecleucel-T (2).
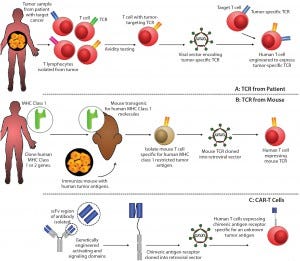
Figure 1: Methods for producing genetically modified T-cells for immunotherapy include (A) T-cell receptor obtained from a patient with the disease; (B) T-cell receptor obtained by immunizing transgenic mice expressing human major histocompatibility complexes (MHCs) with target cancer cells; and (C) a mechanism for producing chimeric antigen receptors.* Following T-cell engineering, resulting cells are expanded ex vivo with interleukin 2 (IL-2) before administration.
The “oncoimmunotherapy wave” includes emerging check-pointinhibitor technology (3) as well as T-cell receptor (TCR) and CAR-T cell technologies (Figure 1). And although it has highlighted the tangible potential for an immunotherapy to reach approval, the attention garnered by these approaches also has underscored the need for significant improvements in bioprocessing technologies and capacities to meet potential and expected market requirements. Critically, commercial-scale production methods — as opposed to those suited to point-of-care and/or blood-processing units — are critical to ensuring commercial sustainability of iTx (4, 5).
Because of the autologous nature of most iTx, however, their manufacturing processes remain highly manual (6), requiring extensive human-operator interaction and decision-making throughout production. Labor costs, inter- and intraprocess consistency, and operator error(s) consequently are major challenges in the effective translation of such therapies from laboratory to commercial scale. For these novel products to succeed, technological innovations will need to enable more seamless transitions between process steps and allow for automation. To achieve those goals, developers will need a strong understanding of the rational path to automation (7) as well as available technology options.
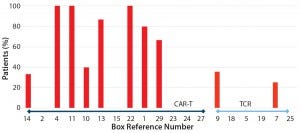
Figure 2: Reported adverse events across 29 studies included in analysis; references cited from the CAR-T and TCR box
Adoptive iTx Clinical Trial Landscape
No comprehensive, quantitative assessment of CAR-T/TCR efficacy or safety has been conducted yet. Here, we provisionally attempt such an assessment by investigating adverse events, target indications, and outcome rates across systematically identified published clinical trials. We
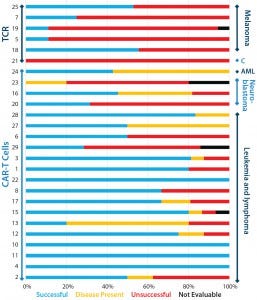
Figure 3: Clinical outcomes of patients after TCR or CAR-T cell therapy; successful outcomes are complete remission, partial remission, or negative for minimal residual disease (MRD); “disease present” means stable disease or MRD positive; unsuccessful outcomes include progressive disease, end of remission, and death. Outcomes for all papers (cited from the CAR-T and TCR box) with reported clinical outcomes are categorized by therapy type (bars on left axis) and cancer type (bars on right axis).
began with a provisional systematic literature review, identifying 29 studies that would be suitable for inclusion in this analysis (see the “CAR-T and TCR” box). Preliminary analysis indicated that to date more trials are using CAR-T cell approaches than genetically engineered TCRs (Figures 3 and 4). In these trials, grades 3 and 4 adverse events appear to be more common with CAR-T cell therapies than with TCRs (Figure 2), although success rates do appear to be greater for the former (Figure 3).
Further research is required for a statistical analysis to be conducted. But it is evident already that demonstrated clinical efficacy is likely to be sufficient to drive significant demand in biomanufacturing capacity that cannot be achieved without a quality by design (QbD) and automated approach. Current biomanufacturing strategies have limited amenability to commercial-scale biomanufacturing scale-up/scale-out. As such, those processes represent a major barrier to industry development that could be alleviated by innovation in bioprocess automation and downstream processing technologies as well as in cell population purification and enrichment.

Figure 4: Stages in systematic automation of an autologous cellular immunotherapy biomanufacturing process
Toward Systematic Automation of an Autologous Cellular iTx
Biomanufacturing Process
To successfully automate a complex biomanufacturing process, a stepped approach provides the most effective solution (Figure 4). In the first phase, developers break down their manufacturing process into separate, sequential steps that each encompass a single function called a unit operation. Next, the development team assesses appropriate equipment for those unit operations and connections between them determine whether linkages are affordable relative to their benefits or demand unjustified expenditures (8). Often, certain breakpoints in a process would be exorbitantly costly to bridge through automation — e.g., morphological assessments and vessel movement between different platforms — yet are easily accommodated through minor operator interactions, which allow for critical-thinking decision points that can be assessed only by human operators (9). Finally, the timing for each process step and its associated equipment costs must be considered. It should not adversely affect equipment use rates, causing integrated components to sit idle when a staged series approach could be more efficient (10). Taking into account unit operations, components, connections, and timelines will ensure that a company ends up with the most robust and affordable iTx manufacturing system with the greatest equipment use rates.
A robust system builds on the basic tenets of automation: removing the human element from bioprocessing and enhancing consistency for both product and process. Clearly, limiting human-operator interactions with a product stream assures higher sterility and reduced opportunities for contamination while also lessening the potential for errors and expanding production hours beyond attended operation. But a common misconception in the industry is that automation confers cost-of-goods (CoG) savings through expedited processing; cost savings usually come from reduced labor needs and the benefits of controlled and reproducible handling (11). Consequently, improved quality and consistency of products made through automation lead to fewer out-of-specification (OoS) results and product losses (12). Closed systems and processes also reduce the risk of contamination.
Taking into account those factors as applied to current iTx processes, it becomes evident that many challenges lie ahead in automating them for commercialization. Methods that originally supported clinician-initiated trials tend to rely primarily if not exclusively on manual techniques and human assessments for cell characteristics requiring further study (13). Additionally, such processes merge equipment from multiple tool providers, making their output communications and connections vital to continuity. And finally, the small process volumes of personalized therapies force highly parallel operations that will be compelled to be cost efficient because of reimbursement constraints (14).
The advantages of implementing automation for the regenerative medicine market are evident and uniquely significant in helping to ensure patient safety. Beyond the improved sterility and consistency of iTx products, online process analytical technology (PAT) allows for monitoring and response to irregularities that arise based on patient differences in a tremendously intricate process. Also, automation supports quality management systems (QMS) in complying with good manufacturing, laboratory, and clinical practices (GMPs, GLPs, and GCPs) and good automated manufacturing practice (GAMP) requirements by mitigating errors and oversights that could foster infractions (15). Furthermore, recent technological advancements are bringing the industry closer to achieving its goal of a more integrated and automated iTx process. Innovations such as liquid-phase detachable selection, activation, and purification technologies are poised to make step-change differences in throughput.
Opportunities to Optimize Immunotherapy Manufacturing
Cell Purification and Enrichment: Purification and enrichment of specific cell subpopulations from heterogeneous starting populations is a critical component of multiple life-science workflows spanning basic cell biology research through clinical-grade cell therapy manufacturing. In all such workflows, a key objective is to obtain a highly purified and viable population of cells for downstream applications, whether in a laboratory or a clinic. For therapeutic applications such as CAR-T and engineered TCR cancer immunotherapies, key objectives include manufacturing efficiency, which requires target-cell purification and processing that are both cost and time efficient.
Cell purification methods currently in widespread use for clinical iTx applications can be broadly classified into two main categories:
physical separation based on properties such as size and density, typically using techniques such as density and counterflow centrifugation
surface-marker–based separations based on using capture ligands and techniques such as magnetic cell separation (MACS) or fluorescence-activated cell sorting (FACS).
Each approach is well established but faces limitations, particularly in terms of unit-operation throughput and equipment use. So alternative solutions are being sought.
Physical separation techniques such as centrifugation are commonly used for prepurification and cell concentration but have low specificity because they separate cells based on sedimentation properties, not by characteristic features or expression of specific markers (16, 17). Centrifugation cannot separate cells with similar sedimentation properties.
Cell purification based on surface markers is dominated by MACS approaches because of their greater scalability in format compared with FACS technology (18). However, currently MACS leaves large numbers of paramagnetic particles bound to separated cells, which is highly undesirable for cell-based therapeutic applications (19). Minimizing the number of attached or internalized beads represents a formidable obstacle here because most products based on magnetic beads lack the ability to readily release bound cells from capture molecules without altering their viability and phenotype. Additionally, the resulting isolated cells require a lengthy culture process to reduce magnetic-bead concentrations to meet clinical release criteria for patients receiving cell-based therapeutic infusions.
Hydrogel Technology: A potential solution to those challenges has come to market recently. A biocompatible phase-change hydrogel can be easily functionalized with cell-capture agents such as antibodies, and it can be dissolved rapidly in a buffer containing a chelating agent (20, 21). (Editor’s Note: See Maribel Rios’s interview with Sean Kevlahan of Quad Technologies in this issue titled “Development of a Novel CellSeparation Platform” for more information.)
As described in the “Fundamental Properties” box, this hydrogel enables liquid-phase cell separation and might solve multiple challenges in cell separation and cell processing workflows. The hydrogel is versatile and can be packed into columns or coated on different substrates such as paramagnetic beads, polymethyl methacrylate (PMMA) acrylic, polydimethylsiloxane (PDMS) silicone, polystyrene, and others, without adverse effect on its differentiate properties.
Fundamental Properties of Phase-Change Hydrogel
Liquid-phase cell chromatography is proving to be highly efficient for the purification and enrichment of specific cell subpopulations such as T lymphocytes from heterogeneous starting populations (22). Successful proof-of-concept experiments have established the potential for functionalized, phase-change, hydrogel-based cell selection and purification using microfluidic devices with paramagnetic particles as carriers for the hydrogel. The phase-change, hydrogel-based approach to cell purification may provide improved cell enrichment and contribute to an overall simplified experimental protocol for cell processing in development of cell therapies. Relative to standard methods, this releasable, liquid-phase technology could solve many inherent challenges of cell separation by safely and rapidly eliminating magnetic particles and achieving high cell recoveries and viabilities through a fast, scalable, and easily executed separation process. Ultimately, it could form a key part of a continuous-flow integrated solution for cell bioprocessing.

Table 1: Comparing separation technologies highlights potential benefits of releasable magnetic particle technology; MACS = magnetic cell separation
Counterflow centrifugation may not be a novel technology itself, but commercial equipment that could process small-scale samples such as autologous immunotherapies would be groundbreaking (Table 1). It would allow for a single system to handle multiple unit operations unit operation while maintaining the most beneficial conditions for stable cell viability. Moreover, the same equipment could be additionally purposed for daily media exchange, debeading, purification, washing, concentration, and infusion into final delivery media. As mentioned above, those steps might otherwise require separation into various suites within a CGMP production facility. However, simplification to one principal piece of equipment would enable operator familiarity and further strengthen process robustness.
Toward Commercialization
CAR-T cell therapies are extraordinarily efficacious and are progressing rapidly through clinical trials, supported by significant investment and adaptive regulatory pathways in both the United States and the European Union. And although the iTx revolution has been a clinician-led “cottage industry” to date, such an environment cannot continue if the field is to realize its commercial promise and reach the maximum number of patients for the greatest possible benefit to society.
Life-science tools and technology industries have understandably sought to respond to the needs of fundamental scientists in academia, who typically work toward integrating all bioprocess steps into self-contained, small-scale manufacturing units. They have predominantly adopted scale-out rather than scale-up purification technologies. Ultimately, however, few of these technologies will be commercially feasible either from a capital expenditure, equipment use, or CoG perspective. In a commercial bioprocess, it is simply infeasible to have a large number of low-throughput and low-use upstream and/or downstream process technologies within a facility. The footprint and calibration burdens are both prohibitive. Online bioprocess control systems including PATs are critical to minimizing process variability and meeting ever-more-stringent regulatory expectations pertaining to current GxPs.
The time is now for applying proven commercial-scale bioprocess equipment and expertise. Building on the work of many clinicians and scientists who have made revolutionary therapies such as CAR-T, TCR, and other iTx possible, the global bioprocessing community must now step forth and respond through robust innovation and quality to enable their availability to all patients in need.
Acknowledgments
We express sincere thanks to the following organizations that have contributed to the CASMI Translational Stem Cell Consortium (CTSCC) as funding and events partners, without whom the consortium and the benefits it will bring to stem cell translation would be constrained: GE Healthcare, the Center for Commercialization of Regenerative Medicine (CCRM), Sartorius Stedim Biotech (formerly TAP Biosystems), Lonza, the California Institute for Regenerative Medicine (CIRM), the Strategies for Engineered Negligible Senescence (SENS) Research Foundation, UK Cell Therapy Catapult, NIH Centre for Regenerative Medicine, the New York Stem Cell Foundation (NYSCF), ThermoFisher Scientific, Eisai, Medipost (US), Medipost (Korea), Celgene, Roche and Oxford BioMedica (UK) Ltd. David Brindley gratefully acknowledges personal funding from the Oxford Musculoskeletal National Institute for Health Research (NIHR), the Saïd Foundation, and the SENS Research Foundation. David Pettitt and James Smith gratefully acknowledge support from the CASMI Translational Stem Cell Consortium (CTSCC). And Smith also gratefully acknowledges support from the UK Medical Research Council and Professor Andrew Carr at the Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences in the University of Oxford’s medical services division.
Conflicts of Interest
This text represents the authors’ individual opinions and may not necessarily represent the viewpoints of their employers. Brindley is a stockholder in Translation Ventures Ltd. (Charlbury, UK) and IP Asset Ventures Ltd. (Oxford, UK), companies that among other services provide cell therapy biomanufacturing, regulatory, and financial advice to pharmaceutical clients. Smith is a consultant with IP Asset Ventures Ltd. Brindley also is subject to the CFA Institute’s codes, standards, and guidelines, so he must stress that this piece is provided for academic interest only and must not be construed in any way as an investment recommendation. Finally, at time of publication, he and the organizations with which he is affiliated may or may not have agreed to and/or have pending funding commitments from organizations named herein.
CAR-T Cell and TCR Cancer Treatment Citations
References
1 CAR-T Cell Therapy Gets Breakthrough Status. Nat. Biotechnol. 32(851) 2014; doi:10.1038/nbt0914-851b.
2 Paediatric Committee. PDCO Monthly Report of Opinions on Paediatric Investigation Plans and Other Activities. European Medicines Agency: London, UK, 25 March 2015.
3 Chen C. Drugmakers Pile into Newest Cancer Treatment Classes: Health. Bloomberg Bus. 11 January 2015.
4 Kaiser AD, et al. Towards a Commercial Process for the Manufacture of Genetically Modified T Cells for Therapy. Cancer Gene Ther. 22, 2015: 72–78.
5 Taga T. Pharmaceutical Companies Race to Build CAR T-Cell Manufacturing Processes. Gen. Eng. News 3 August 2015.
6 Brindley DA, et al. The Potential Application of Real-Time Release Testing for the Biomanufacture of Autologous Cell-Based Immunotherapies. BioProcess Int. 13(4) 2015: S34–S43.
7 Brindley DA, Wall I, Bure K. Automation of Cell Therapy Biomanufacturing. BioProcess Int. 11, 2013: S18–S25.
8 Stone E. Technological Advances Facilitate Autologous Cell Therapy Scale-Out. Gen. Eng. News 25 August 2015.
9 Ratcliffe E, Thomas RJ, Williams DJ. Current Understanding and Challenges in Bioprocessing of Stem Cell-Based Therapies for Regenerative Medicine. Br. Med. Bull. 100, 2011: 137–155.
10 Jenkins M, et al. Patient-Specific hiPSC Bioprocessing for Drug Screening: Bioprocess Economics and Optimisation. Biochem. Eng. J. 2016: in press.
11 Hourd P, et al. Manufacturing Models Permitting Roll Out/Scale Out of Clinically Led Autologous Cell Therapies: Regulatory and Scientific Challenges for Comparability. Cytother. 16, 2014: 1033–1047.
12 Thomas RJ, et al. Automated, SerumFree Production of CTX0E03: A Therapeutic Clinical Grade Human Neural Stem Cell Line. Biotechnol. Lett. 31, 2009: 1167–1172.
13 Coopman KM, Medcalf N. From Production to Patient: Challenges and Approaches for Delivering Cell Therapies. StemBook March 2014; www.stembook.org/node/6149.html.
14 Scott C, Rios M. BioProcess Theater: Clinical and Commercial Manufacturing. BioProcess Int. 13(5) 2015: S29–S32.
15 GAMP5: The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture. International Society for Pharmaceutical Engineering: Tampa, FL, 2008.
16 Ren Y, et al. Isolation, Expansion, and Differentiation of Goat Adipose-Derived Stem Cells. Res. Vet. Sci. 93, 2012: 404–411.
17 Bunnell BA, et al. Adipose-Derived Stem Cells: Isolation, Expansion and Differentiation. Methods 45, 2008: 115–120.
18 Placzek MR, et al. Stem Cell Bioprocessing: Fundamentals and Principles. J. Roy. Soc. Interface 6, 2009: 209–232.
19 Diogo MM, Da Silva CL, Cabral JM. Separation Technologies for Stem Cell Bioprocessing. Biotechnol Bioeng. 109, 2012: 2699–2709.
20 Armstead A. A Novel Hydrogel-Based Approach for Cell Isolation, Purification and Release. Amer. Lab. 22 February 2016. www.americanlaboratory.com/914-Application-Notes/183423-A-Novel-Hydrogel-Based-Approach-for-Cell-Isolation-Purification-and-Release.
21 Hatch A, Pesko DM, Murthy SK. Tag-Free Microfluidic Separation of Cells Against Multiple Markers. Anal. Chem. 84(10) 2012: 4618–4621; doi:10.1021/ac300496q.
22 Zhu B, et al. Microfluidic Isolation of CD34-Positive Skin Cells Enables Regeneration of Hair and Sebaceous Glands In Vivo. Stem Cells Transl. Med. 3(11) 2014: 1354–1362; doi:10.5966/sctm.2014-0098.
Further Reading
Cruz CRY, et al. Infusion of Donor-Derived CD19-Redirected Virus-Specific T Cells for B-Cell Malignancies Relapsed After Allogeneic Stem Cell Transplant: A Phase 1 Study. Blood 122, 2013: 2965–2973.
Davila ML, et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med. 6, 2014: 224ra25.
Grupp SA, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 368, 2013: 1509–1518.
Hollyman D, et al. Manufacturing Validation of Biologically Functional T Cells Targeted to CD19 Antigen for Autologous Adoptive Cell Therapy. J. Immunother. 32, 2009: 169–180.
Jensen MC, et al. Antitransgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Redirected T Cells in Humans. J. Am. Soc. Blood Marrow Transplant. 16, 2010: 1245–1256.
Jensen MC, et al. Human T Lymphocyte Genetic Modification with Naked DNA. Mol. Ther. J. Am. Soc. Gene Ther. 1, 2000: 49–55.
Johnson LA, et al. Gene Therapy with Human and Mouse T-Cell Receptors Mediates Cancer Regression and Targets Normal Tissues Expressing Cognate Antigen. Blood 114, 209: 535–546.
Kalos M, et al. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci. Transl. Med. 3, 2011: 95ra73.
Kalos M, June CH. Adoptive T Cell Transfer for Cancer Immunotherapy in the Era of Synthetic Biology. Immunity 39, 2013: 49–60.
Kochenderfer JN, et al. Eradication of B-Lineage Cells and Regression of Lymphoma in a Patient Treated with Autologous T Cells Genetically Engineered to Recognize CD19. Blood 116, 2010: 4099–4102.
Laport GG, et al. Adoptive Transfer of Costimulated T Cells Induces Lymphocytosis in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma Following CD34+Selected Hematopoietic Cell Transplantation. Blood 102, 2003: 2004–2013.
Lee DW, et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet 385, 2015: 517–528.
Leen AM, et al. Monoculture-Derived T Lymphocytes Specific for Multiple Viruses Expand and Produce Clinically Relevant Effects in Immunocompromised Individuals. Nat. Med. 12, 2006: 1160–1166.
Maude RJ, et al. The Last Man Standing Is the Most Resistant: Eliminating Artemisinin-Resistant Malaria in Cambodia. Malar. J. 8, 2009: 31.
Micklethwaite KP, et al. Derivation of Human T Lymphocytes from Cord Blood and Peripheral Blood with Antiviral and Antileukemic Specificity from a Single Culture as Protection Against Infection and Relapse After Stem Cell Transplantation. Blood 115, 2010: 2695–2703.
Milone MC, et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. J. Am. Soc. Gene Ther. 17, 2009: 1453–1464.
Moher D, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6, 2009: e1000097.
Pule MA, et al. Virus-Specific T Cells Engineered to Coexpress Tumor-Specific Receptors: Persistence and Antitumor Activity in Individuals with Neuroblastoma. Nat. Med. 14, 2008: 1264–1270.
Savoldo B, et al. CD28 Costimulation Improves Expansion and Persistence of Chimeric Antigen Receptor-Modified T Cells in Lymphoma Patients. J. Clin. Invest. 121, 2011: 1822–1826.
Son D, et al. The Effect of Centrifugation Condition on Mature Adipocytes and Adipose Stem Cell Viability. Ann. Plast. Surg. 72, 2014: 589–593.
Tran CA, et al. Optimized Processing of Growth Factor Mobilized Peripheral Blood CD34+ Products By Counterflow Centrifugal Elutriation. Stem Cells Transl. Med. 1, 2012: 422–429.
Tumaini B, et al. Simplified Process for the Production of Anti-CD19-CAR-Engineered T Cells. Cytother. 15, 2013: 1406–1415.
Kim Bure is director of regenerative medicine, and Franziska Faulstich is regenerative-medicine product manager at Sartorius Stedim (Göttingen, Germany). Andrew Ball is vice president of research and development, and Kiara Biagioni is a marketing product manager at Quad Technologies (Woburn, MA). Sunil Mehta is president and chief executive officer of kSep Systems (Morrisville, NC). Rajan Choudhary is an undergraduate researcher at the Oxford–UCL Centre for the Advancement of Sustainable Medical Innovation (CASMI). Zeeshaan Arshad is an undergraduate student at CASMI, at the University of St. Andrews School of Medicine (St. Andrews, UK). David Pettitt is a doctoral researcher at CASMI and in the medical sciences division’s department of pediatrics at the University of Oxford, UK. Georg Holländer is an action research professor in the medical sciences division’s department of pediatrics at the University of Oxford and the Weatherall Institute of Molecular Medicine at John Radcliffe Hospital (Oxford, UK). Hussein Al-Mossawi is a lecturer in rheumatology and musculoskeletal sciences at the University of Oxford’s Nuffield Department of Orthopedics (Oxford, UK). Brock Reeve is executive director of the Harvard Stem Cell Institute (Cambridge, MA). Corresponding author James Smith is a CASMI research associate and professor in the University of Oxford’s Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences; [email protected]. And David Brindley is founder and academic director of CASMI’s Translational Stem Cell Consortium, senior healthcare translation research fellow in the medical sciences division’s department of pediatrics at the University of Oxford, Cooksey-Saïd fellow in healthcare translation at the University of Oxford’s Saïd Business School, honorary senior research associate at the University College London School of Pharmacy’s Centre for Behavioral Medicine (London, UK), a research fellow in cell therapy commercialization at the Harvard Stem Cell Institute, and regenerative medicine regulation and risk management lead at the USCF-Stanford Center of Excellence in Regulatory Science and Innovation (CERSI) in Stanford, CA.
You May Also Like





