Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Thermo Fisher Scientific
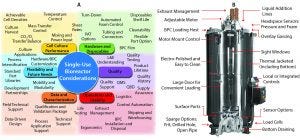
Figure 1: Highlights of requirements and features to be considered during single-use bioreactor selection and evaluation process; (A) highlight of key concepts for consideration; (B) features of the Thermo Fisher Hyperforma 2,000 L S.U.B.
There is ever increasing pressure for the biopharmaceutical industry to drive toward higher efficiency and lower costs. Compared to the past, target markets for many drugs typically are becoming smaller, and so-called blockbuster drugs are becoming more the exception than the rule. Regulatory agencies have continued to increase the pressure on drug makers to meet increasing quality standards and accept higher levels of responsibility. Furthermore, customer pricing, healthcare markets, and recent biopharmaceutical pricing scandals all add incentives toward more efficient processes.
Naturally, improving operational efficiency includes many aspects, including but not limited to drug discovery, preclinical testing, process development, clinical manufacturing, clinical trials, and commercialization. Bioprocessing is an integral part of biotherapeutic development operations and thus can directly affect operational efficiency.
Single-use technologies (SUTs) are a propitious option for creating low-cost, efficient, and flexible bioprocesses capable of streamlining development stages from process development through commercial manufacturing (1). SUTs are generally modular, allowing for flexible systems to be easily exchanged or integrated into platform processes, tailoring them to specific drug candidates. This flexibility — along with the potential to create completely closed systems — enables a single manufacturing or development site to run multiple drugs either in succession or potentially in parallel. Truly closed systems also can reduce the environmental control grade required. The ability to be closed, flexible, and in parallel are key ingredients for nimble scheduling and manufacturing to maximize production efficiencies.
Using SUTs generally mitigates risks associated with high upfront costs of stainless steel (SS) plant development. Like SS, SUTs can be scaled-up. SUTs have the additional benefit easily being scaled-out. For example, during preclinical and clinical stages, manufacturing might require only a single 2,000 L bioprocess for necessary cell cultures. As product demand increases with market growth, that bioprocess might be expanded effectively by process duplication within the same site or at a new site in 6–9 months. Scale-out is particularly useful toward derisking drug-product commercialization growth and streamlining the manufacturing of products in specific regulatory markets (e.g. CFDA, USFDA, EMA).
Upstream Processing: First Critical Step
In bioprocessing from bench to manufacturing scale, the first critical bioprocessing step is upstream, which includes cell expansion and full-scale culture. Typically, this process proceeds in incubators and scale into bioreactors. As the first step in the bioprocessing workflow, bioreactor performance and quality can readily impact each subsequent bioprocess.
Single-use bioreactors (S.U.B.s) developed out of technology of flexible single-use film containers used for storing liquid media and biopharmaceutical materials. As the feasibility and ease-of-use of the first single-use containers became apparent, Thermo Fisher Scientific saw the potential of replacing standard SS bioreactors with single-use options as a method to eliminate the need for clean-in-place (CIP) and steam-in-place (SIP) steps while simultaneously reducing validation requirements, cross-contamination risks, operational costs, total footprint, and initial investments.
The first 50 L and 250 L S.U.B. systems were launched in 2006 and were the first S.U.B units on the market, with further launches of S.U.B.s up to 2,000 L size, single-use mixers (S.U.M.s) from 50 L to 2,000 L, and single-use fermentors (S.U.F.s) at 30 L and 300 L scales. This extensive experience with SUTs has helped solidify Thermo Fisher Scientific as the leader within the SUT bioprocessing workspace. With the largest 2,000 L S.U.B installation base, Thermo Fisher Scientific has insight into the evolving needs of bioprocessing and continually strives to improve key attributes of its Hyperforma S.U.B. through technologic innovations, which enable flexibility and customization for each specific process.
The numerous design variables for S.U.B. systems (highlighted in Figure 1A) and the complex interaction of these variables can be a potential stumbling block for bioreactor design and S.U.B. selection potential challenges. In this review series, we review the key attributes to consider during S.U.B evaluation and selection and present related key data regarding Thermo Fisher Scientific’s Hyperforma S.U.B.s. In this first section of the series, we focus on reactor performance to control critical process parameters.
Controlling Critical Process Parameters Through Performance
Reproducible, effective, and efficient upstream bioprocesses generally rely on identifying critical process parameters (CPPs) and using a bioreactor that can achieve good control over those parameters. CPPs in upstream processes typically include pH, dissolved oxygen (DO), and temperature. Other possible CPPs are viable-cell density, culture viability, specific growth rate, nutrient levels, and other measurable parameters. Key mechanisms to control the primary CPPs are based in bioreactor performance: mixing times and efficiency, total power input per fluid volume (PIV), and mass transfer efficiencies/rates (kLa). These attributes are in turn most directly affected by bioreactor design variables: 1) impeller type, count, speed, and location; 2) reactor geometry, jacket, and material; and 3) sparging mechanisms. Careful consideration of reactor design enables these interacting variables to be leveraged for maximum effect.
Accurate and reliable measurement of CPPs is the first step toward controlling them properly. For each CPP, different options and vendor offerings are available — from single-use probes to conventional ones. Understanding the performance attributes of these probes is necessary for ensuring reliable performance for your process. Thermo Fisher Scientific can provide guidance for probe selection based on internal qualification and validation testing. However, Thermo Fisher retains an open platform with respect to probes selection, and the final selection remains the decision of a consumer according to their needs. The number and type of probes available for use are also flexible, with 10+ ports allowed for probes in the face of a reactor across 50 L to 2,000 L scales. Combining the flexibility and customizability allows Thermo Fisher S.U.B s to meet current and future process analytical technology (PAT) needs.
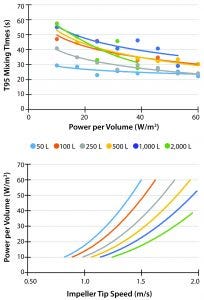
Figure 2: Mixing times and power per volume; (A) T95 mixing times of saline solution; solution was loaded at liquid surface and measured for homogeneity across column height and reported by slowest response time; (B) calculated power per volume with respect to tip-speed
CPP homogeneity throughout the cell culture volume is essential to ensure high quality, reproducible upstream bioprocesses. Rapid mixing of vessel volumes helps prevent formation of gradients (e.g., pH, temperature) that would form if fluid were poorly mixed. For example, poorly mixed fluid with strong base addition can result in unacceptable pH spikes at the location of addition. Generally, higher power per volume results in better mixing. Additional factors that affect mixing include impeller design and reactor geometry. Thermo Fisher Hyperforma S.U.B.s maintain good mixing across vessel scales by implementing carefully conserved vessel geometry and impeller selection (T95 <50 s for P/V ≥ 20 W/m3) (Figure 2A).
Although power input improves mixing, there is a point of trade-off, where the shear stress generated by impeller-tip speed becomes sufficient to damage cells in culture. While exact sensitives to shear and tip speed are unique to each cell line, heuristic guidelines keep tip speed between 1 m/s and 3 m/s. Furthermore, the more uniformly tip speed can be maintained across scales, the more representative scale-down models become. Thermo Fisher S.U.B.s provide tip speeds within the heuristic guidelines while still providing acceptable mixing (Figure 2B).
Another aspect of controlling CCPs is mass transfer. The dynamic respiration of cells in culture necessitates mass transfer. Frequently, consideration of mass transfer focuses predominantly on moving oxygen into solution with monitoring inline through the dissolved oxygen (DO) probe. Higher cell densities demand more oxygen and require improved mass transfer. A further, (easily overlooked) aspect of mass transfer is removal of respired carbon dioxide (CO2). Carbon dioxide build-up can be deleterious to bioprocess consistency by affecting pH, buffering capacity, metabolic processes, and product quality.
Mass transfer is achieved through two primary avenues: 1) sparging into the headspace above the liquid and 2) sparging gasses through the bottom of a vessel. Headspace sparge or sweep plays a role in mitigating the potential buildup of potentially deleterious gasses (e.g., CO2 or NH3) above the liquid surface. For a vessel operated at or near its maximum volume, a standard headspace sweep through an open port typically is sufficient to remove these gasses. However, at lower volume operations (e.g., 5:1 turn down or 20% operational volume), a standard headspace sweep can become ineffective, and toxic gasses can build up above the liquid’s surface, affecting parameters such as pH and metabolic pathways. Thermo Fisher Cross-Flow Sparge (CFS) technology was developed to eliminate the risk of undesirable gas buildup by including an additional headspace sparge for low-volume operations and has been proven effective (2).
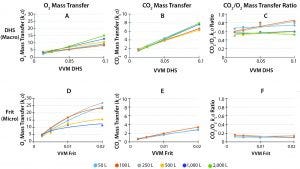
Figure 3: Mass transfer coefficients for O2 and CO2 using Thermo Fisher’s precision drilled-hold sparger (DHS) (macro-sparge) and polymer-sintered frit (micro-sparge); (A, B) oxygen transfer coefficients across volume-normalized sparge rates (vessel volumes per minute (VVM) gas flow rate); Thermo Fisher’s DHS is engineered for scalability for oxygen (A) and carbon dioxide (C) transfer across volumes at given VVM by taking into account key sparger attributes such as bubble size, bubble count, and residence time. By keeping the CO2/O2 transfer ratio >50% (e), the DHS helps ensure sufficient CO2 stripping. The microsparge of the frit provides high oxygen transfer (B) yet scales poorly due to physical limitations of flows and bubble-size with respect to bubble-residence time. Furthermore, the frit CO2 transfer coefficient (D) and CO2/O2 ratio (F) are typically insufficient for CO2 stripping. Using a combination of DHS and frit allows for excellent control of dissolved oxygen and ensures sufficient CO2 stripping from the reactor; reported at ≈20 W/m3 in standard configurations.
Bottom sparger options traditionally include frits (micro), open-pipe sparges (OPS) (macro), and drilled-hole spargers (DHS). Frits typically provide efficient oxygen delivery per flow rate (Figure 3D). However, frits exhibit poor uniformity of performance across scales — particularly at high flow rates, primarily due to restrictions in flow rate maximums and bubble size. Lack of scalability obfuscates scale-down processing models. A further, more challenging issue is that the small size and low flow of bubbles from frits have a low capacity for removing CO2 (Figure 3E), particularly with respect to oxygen (O2) delivery (Figure 3F). This poor transfer ratio between carbon dioxide and oxygen can lead to processes oversaturated with carbon dioxide.
One approach to assist in removal of carbon dioxide buildup is to use an open-pipe sparge (OPS). These provide high CO2/O2 transfer ratio, often ranging from 1 to 2 (compared to frit range ~0.15). However, OPSs are ~5× less efficient per flow rate than frit-spargers at O2 and CO2 delivery, resulting in dramatically higher flows required for CO2 removal. The potentially high flows from OPSs can lead to further challenges, such as development of deleterious shear rates due to gas jetting at the OPS and the formation of foams stabilized with large OPS bubbles and small frit bubbles (3).
An alternative approach to more balanced mass transfer is DHS technology. Thermo Fisher Scientific has invested extensive research into the development of its proprietary, light-weight DHS system to enable high O2 delivery and maintain high CO2/O2 transfer ratios (Figure 3A–C). A DHS is designed with a 2× safety factor to prevent damaging gas exit velocities that might be seen with OPSs. Furthermore, this engineered DHS enables unparalleled scalable performance, allowing for near-uniform mass transfer characteristics from 50 L to 2000 L with respect to flow per volume (VVM) (Figure 3A–C). Such scaling is essential for simplifying technology transfer between process development and manufacturing and reduce the need for costly large-scale engineering runs prior to commercial-scale manufacturing. Thermo Fisher Scientific DHS technology provides the operator with exquisite control over mass transfer, helps ensure sufficient CO2 removal, and can be combined with frit technology to achieve best-in-class oxygen delivery for high-intensity cultures.
Temperature control is another key to improving bioprocess performance. Stable temperature control throughout a culture is a necessity and typically not difficult to accomplish with appropriate controllers. More challenging user requirements usually occur during media heat-up and culture cool-down, which typically have specified operations time windows to reduce operations time and protect products’ critical quality attributes (CQAs). Options for temperature control typically are accomplished through vessel jacketing and can include resistive heating elements, which lack the ability to cool beyond ambient conditions. For jacketed vessels, performance is related directly to jacketed surface area with respect to volume: In general, the larger the jacketed area, the better performance. Jacketing the bottom of vessels maximizes jacketed area and improves control at low-volume operations. For this reason, Thermo Fisher Scientific Hyperforma S.U.B.s have jacketed bottoms and maximized vessel-wall jacketing for maximum temperature control, even at low volumes.
A final aspect of temperature control performance is selecting appropriate temperature control units (TCUs). TCUs combined with jacket design synergistically determine temperature control performance. Thermo Fisher and Finesse have extensive experience in identifying appropriate temperature control configurations for a given bioprocess setup.
Further and Future Considerations
Maintaining CPPs through S.U.B. performance (e.g. mass transfer, power per volume, temperature control) is naturally a critical consideration during selection of S.U.B.s. There are, however, further considerations beyond this essential performance. In part 2 of this article, we will examine some of these further considerations, focusing on the physical and operational aspects of S.U.B.s, such as S.U.B. hardware ergonomics, bioprocessing container flexibility, and quality vendor relationships. As the biopharmaceutical industry continues towards streamlined bioprocessing and cell-culture biology intensifies, selection criteria of S.U.B.s and other bioprocessing technology will become increasingly rigorous, emphasizing the importance of considering every aspect of technologies under evaluation.
Acknowledgments
The authors would like to acknowledge the many contributors to the data and discussion provided in this work, including Christopher Brau, and Jordan Cobia for their assistance in data collection and analysis, and the many industry collaborators who provide the essential industrial perspective.
References
1 Pollard D, et al. Standardized Economic Cost Modeling for Next-Generation MAb Production. BioProcess Int. 14(8) 2016: 14–23.
2 Madsen B, et al. Simplifying Upstream Process Development and Scale-Up: Single-Use 5:1 Turndown-Ratio Bioreactor Technology. BioProcess Int. 15(11) 2017: 47–50.
3 Zhu Y, et al. NS0 Cell Damage by High Gas Velocity Sparging in Protein-Free and Cholesterol-Free Cultures. Biotechnol. Bioeng. 101, 2008: 751–760; doi:10.1002/bit.21950.
Corresponding author Dr. Mark T. Smith ([email protected]) and Dr. Tony W. Hsiao are senior engineers in the BioProcessing Single-Use Technologies Advanced Technology Research Group at Thermo Fisher Scientific. Dr. Benjamin Madsen is senior engineer in the BioProcessing Single-Use Technologies Process Development Group at Thermo Fisher Scientific. Nephi Jones is the senior manager over applications research in the Bioprocessing Single-Use Technologies R&D at Thermo Fisher Scientific.
For further information regarding the Thermo Fisher Hyperforma S.U.B. system or other Thermo Fisher SUT products, please visit our website at
https://www.thermofisher.com/single-use-technologies or contact the authors.
© 2018 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
You May Also Like