WWW.GETTYIMAGES.COM
Vaccines against sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are designed to elicit immune responses that prevent recipients from getting — or becoming seriously ill or dying from — novel coronavirus disease (COVID-19). Two available COVID-19 vaccines are based on genetically engineered messenger RNA (mRNA). After administration, such molecules give muscle cells “instructions” for how to make target proteins — e.g., the SARS-CoV-2 spike (S) protein. Immune-system detection of those proteins prompts creation of neutralizing antibodies. Immediately upon delivery of the instructions, mRNA is broken down. It never enters the nucleus, where a cell keeps its DNA.
Chinese biopharmaceutical company Walvax Biotechnology Co., Ltd., is helping to advance the global development of mRNA vaccines to fight current and future COVID strains. With Suzhou Abogen Biosciences, the company obtained regulatory approval in 2021 to conduct phase 3 clinical trials for its ARCoV mRNA vaccine in ...
The COVID-19 pandemic has become the most recent reminder of how accessibility to drugs and vaccines depends heavily on geographical location and a country’s economic health. Some countries continue to lack the infrastructure, regulatory support, and trained workforce necessary for establishing a viable bioindustry. Unizima, part of the Univercells Group, is a team of experts, scientists, and engineers that partners with public and private companies and other organizations to help build biomanufacturing capabilities in low- and middle-income countries (LMICs).
We spoke with Hala Audi (chief executive officer at Unizima) and David Honba (head of business development at Unizima) about how the company is addressing the limitations and difficulties of working in such regions. To her current position, Audi has brought her experience in construction and infrastructure procurement in government and her experience in UK government policy on global health and antimicrobial resistance. Honba is an experienced biote...

 +2
+2https://www.stock.adobe.com
The COVID-19 pandemic has demonstrated the importance of — and significant demand for — vaccines. However, vaccine development for large-scale manufacturing can be difficult and resource intensive because of the diversity and complexity of vaccine types that are needed. Developing a new vaccine typically takes more than a decade, with costs ranging from US$200 million to $500 million per successful program. That figure rises to $8 billion for epidemic vaccines (
1
). Vaccine programs also face a 90% risk of failure (
2
).
The Inno4Vac project intends to accelerate and minimize risks for vaccine development by tackling some major bottlenecks and creating innovative methods to identify the most promising vaccine candidates at early development stages. The Inno4Vac consortium is a public–private partnership established with financial support from the Innovative Medicines Initiative 2 (IMI-2), a joint undertaking of the European Union and the European Federation of Pharmaceutical I...

 +5
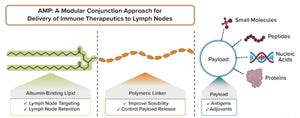
+5Subunit vaccines stimulate immune cells by delivering selected components of a pathogen of interest rather than the entire pathogen. Elicio Therapeutics is developing subunit vaccines that target a recipient’s lymph node to elicit a robust immune response. The vaccines are based on the company’s amphiphile (AMP) platform (Figure 1). The platform-based drug candidates are targeting cancer and COVID-19. Their development would allow the vaccine to be stored without the need for ultracold and cold storage.
Figure 1:
The amphiphile platform enables lymph node delivery of validated therapeutics with modular application.
Below, we discuss the development of Elicio Therapeutics’s vaccine candidates and AMP platform with Peter DeMuth, PhD (chief scientific officer) and Chris Haqq, MD, PhD (executive vice president, head of R&D and chief medical officer at Elicio Therapeutics).
Vaccine Development
What drove Elicio to work with subunit vaccines?
Haqq:
It’s a bit of a misnomer to call our vaccines traditional sub...

 +2
+2Desentum, a biopharmaceutical company that specializes in developing allergen immunotherapy (AIT) products, has partnered with contract development and manufacturing organization (CDMO) Biovian to advance hypoallergens based on targeted modifications to recombinant proteins. I spoke with Kati Sallinen (director of strategy and communications at Desentum) and Jonne Vaarno (project manager at Biovian) about their companies’ development of AITs and a potential platform to treat different types of allergies.
Our Discussion
We all know someone with an allergy, and many people have more than one. What’s the extent of allergies as a global health problem?
Sallinen:
The prevalence of allergies has increased for decades, to an extent that it is sometimes called an “allergy epidemic.” In the European Union, for example, more than 150 million people suffer from allergic rhinitis, asthma, or food allergies. The burden of allergies is social, economic, and medical. Severe allergies reduce quality of life, limit socia...

 +1
+1