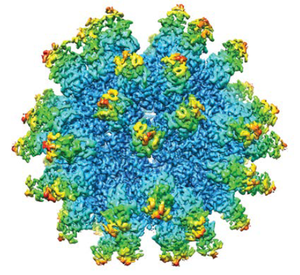
Anelloviruses are icosahedral virions that encapsdate small single-stranded DNA genomes. (
HTTPS://RINGTX.COM
)
Viruses are ubiquitous members of every ecosystem, including the human body (
1
). A common misconception is that all viruses are harmful. In fact, their relationships to human hosts can be pathogenetic, symbiotic, or commensal. In the latter case, a virus benefits from but neither harms nor helps its host (
2
). Commensal viruses are as abundant as human cells, and their coexistence has persisted for millennia, permitting our immune systems to recognize them as part of us. Among the most abundant members of the human commensal virome are anelloviruses (
3
). The
Anelloviridae
family is believed to exist inside every healthy human being from birth, having been shared maternally through placental blood, and such viruses persist throughout a human host’s life (
4–6
).
Anelloviridae
comprises more than 30 genera (
7
) characterized by icosahedral virions that encapsidate a small single-stranded ...
Early decisions can impact the success of a novel gene therapy, so how can therapeutics companies make sure to set off on the right track?
In this article, drug developers will learn some things that they should consider at the start of their therapeutic journey. We explore the impact of early technical decisions, including the importance of seeking high-yielding plasmids and a well-validated cell line. We cover logistical considerations, such as plasmid supply chain challenges and how to avoid them. Finally, we highlight the long-term benefits of forming a strategic partnership with a highly-experienced CDMO early in the therapeutic journey.
Fill out the form below to read the full article from WuXi Advanced Therapies.
Immunogenicity is the ability of a substance, such as a foreign and/or potentially dangerous protein, to provoke an antigen-specific immune response. However, some immune responses can be detrimental, such as in autoimmune diseases and unwanted reactions to biological therapeutics. The latter case can compromise biopharmaceutical safety and efficacy, and preexisting immunity against biologic components can preclude patients from receiving life-changing treatments, perhaps most notably in gene therapy (
1
).
Gene therapies are designed to target the root cause of a genetic disease by gene replacement, addition, inhibition, or editing (
2
). Often, such treatments are delivered by viral vectors. The current standard for in vivo gene delivery is adenoassociated virus (AAV), which was detected in humans in the 1960s (3). AAV is the most widely applied gene therapy vector for several reasons. It can transduce host cells and shows robust, long-term transgene expression, but it is largely unable to integrate int...
The Regenxbio Manufacturing Innovation Center in Rockville, MD.
Demand for gene therapies based on adenoassociated virus (AAV) vectors continues to exceed manufacturing capacity. Part of the imbalance stems from the growing number of AAV-based candidates that are advancing through clinical studies. Zhao et al. report that, in September 2021, researchers were enrolling participants for and/or conducting 137 trials for such products (
1
). As of August 2022, three AAV-based therapies have received commercial authorization in the United States and/or European Union, and other products have received conditional approval (
2–5
). Without a doubt, the COVID-19 pandemic has exacerbated preexisting shortages by compelling biopharmaceutical companies to direct considerable labor, research, and manufacuring resources to developing SARS-CoV-2 vaccines and antivirals. Several contract development/manufacturing organizations (CDMOs/CMOs) have pledged to install new manufacturing capacity to address current shortages (
Therapeutic developers face significant challenges in purifying cell and gene therapies (CGTs). Current technologies for laboratory-scale lentivirus (LV) feedstock preparation are inefficient and not fit for purpose. From the benchtop to the clinic, all stages of CGT development require new solutions that break away from the current paradigm of biopharmaceutical manufacturing.
Nereus LentiHERO nanofiber-based technology can address difficulties associated with purifying large and fragile modalities. Here, experts from the viral vector team at Astrea Bioseparations discuss how Nereus LentiHERO technology enables laboratory-scale lentiviral vector (LVV) purification by addressing industry needs for increased throughput and improved viral particle recovery.
Special Considerations for Lentivirus
How do LV characteristics influence their purification?
Ian Scanlon (viral vector downstream processing):
LVVs differ in several respects from other viral vectors used in CGT. Whereas adenoassociated virus (AAV) caps...

 +1
+1HTTPS://ALAMY.COM
According to the US Food and Drug Administration’s (FDA’s) process validation guidance, critical quality attributes (CQAs) and critical process parameters (CPPs) are used to assess the statistical stability of a bioprocess and its ability to meet acceptable criteria as a part of a continued process verification (CPV) program using control charts (
1
). For those control charts, control limits are used to assess the statistical stability of process parameters and attributes. When data are normally distributed, control limits are established straightforwardly by placing control limits three standard deviations away from an average (mean) value (
2
).
However, bioprocess data often are not normally distributed, so a regular I-chart with control limits placed three standard deviations from a mean value cannot be used. Such data include
•
censored data
, which are partially known and often are reported as being below the limit of quantification (LoQ)
•
trending mutually
exclusive events
...