Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Single-use filtration systems are increasingly replacing traditional stainless steel filter assemblies, piping, and tanks for purification and storage of bioprocess fluids in biopharmaceutical manufacturing. Unfamiliarity with polymeric materials and the need to ensure patient safety, however, have made extractables and leachables from these new components and systems a primary concern of process developers along with specialists in quality, validation, regulatory affairs, as well as agency reviewers.
A general risk-based approach to determination of extractables and leachables from disposable bioprocess equipment has been published by the Bio-Process Systems Alliance (BPSA, www.bpsalliance.org). Detailed characterization of extractable compounds in model solvents — or leachables in drug product streams — can be generated through the use of sophisticated analytical equipment. Extractables studies show the potential for a material to contribute leachable substances into a drug product or process fluid. Resulting data provide an important platform on which decisions can be made regarding the need for (or extent of) actual testing for leachable substances in process fluids or final drug formulations. Because extractables data can be generic based on extractions in model solvents under exaggerated conditions that bracket the actual processes conditions, they can be generated by suppliers and then made available to end users for incorporation in their own test program assessments and regulatory submissions.
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: UPSTREAM AND DOWNSTREAM PROCESSING
WHO SHOULD READ: PRODUCT/PROCESS DEVELOPMENT, QA/QC, AND ANALYTICAL PERSONNEL
KEYWORDS: ANALYTICAL METHODS, PROCESS VALIDATION, DISPOSABLES
LEVEL: INTERMEDIATE
In a previous publication, we reported extractables studies on single-use sterile connectors and filter capsules using model solvents under worst-case conditions (1). Our results showed that water and ethanol extractables from connectors were extremely low, often below the detection limits, and consequently were unlikely to contribute significant leachables into pharmaceutical products. For capsule filters with high–surface-area membranes, higher levels of water and ethanol extractables were obtained, as expected, and the composition was determined to be consistent with the filter materials of construction.
Figure 1:
PALL LIFE SCIENCES (WWW.PALL.COM)
Both capsule filter and sterile connector materials were evaluated by their supplier for biological safety during core validation of the products. It was concluded that such extractables data could be an important part of risk assessment by users for their potential leachable materials in final drug products from single-use process components and could also be incorporated into process validation documentation. This type of approach has long been successfully applied in regard to final sterilizing filters for aseptic processing.
Here we report similar studies of flexible tubing and polymeric film biocontainers. Coupling these results with our previously reported data for filter capsules and sterile connectors can help users of disposable multicomponent systems build up a qualitative and quantitative extractables/potential leachables profile of their total systems. It can then be of value in developing appropriate SOPs to minimize actual leachables from single-use process equipment in final drug product formulations and containers and provide validation support.
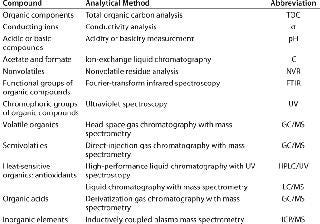
Analytical Methods: Today’s advanced analytical instrumentation offers suppliers and users the opportunity not only to identify the qualitative nature of extractable materials in great detail, but also to quantify them at very low concentrations, often down to parts per billion (ppb) or even parts per trillion (ppt) levels. The resulting information can increase the accuracy of risk assessment of extractables as potential leachables in biopharmaceutical manufacturing processes and final drug products (2,3,4,5). We used 13 different analytical methods (Table 1) to identify and quantify extractables from flexible tubing and polymeric film biocontainers.
Table 1: Extractable compounds and applicable analytical methods
Flexible Tubing
Although the contact time for flexible tubing is typically short (less than a day), and the diameter/circumference of the tubing is relatively small, the total length used in disposable systems can be extremely large, sometimes over 100 m as in the case of extensive tubing manifolds. Significant quantities of materials leached from tubing could therefore be present in a process fluid despite the relatively short process time.
To assess that potential, we performed an extractables study on a thermoplastic elastomer tubing after gamma irradiation to 50 kGy using appropriate model solvents under exaggerated conditions. Such tubing is commonly used in single-use systems because of its ability to be melt-welded, unlike silicone tubing.
Table 2 summarizes the tubing properties and extraction conditions we used. Pretreatment by gamma irradiation at 50 kGy and an extraction procedure of continuous contact and agitation for 72 hours represented an exaggerated condition compared with typical process operations.
Table 2: Tubing* properties and extraction conditions

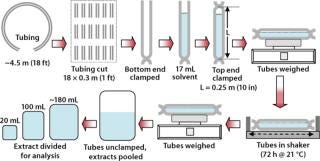
Tubing Extraction Procedure: Figure 1 is a schematic of our procedure for extraction of flexible tubing. The 18 segments of tubing constituted one set, and we combined extracts from each segment for analysis. Two sets of tubing were extracted, and we calculated the mean values for the results presented below. The negative control was solvent in a polytetrafluoroethylene (PTFE) bottle, and we obtained a positive control by dissolving 20 mg of potassium hydrogen phthalate (KHP) in the test solvent and evaporating the resulting solution to determine KHP recovery. We confirmed its chemical identity using Fourier-transform infrared (FTIR) spectroscopy with known standards for comparison.
Total Organic Carbon (TOC), Conductivity, pH, and Acetate/Formate: Table 3 presents the results for TOC, conductivity, pH, and acetate/formate of extracts with deionized (DI) water. These extracts showed low TOC levels (<10 ppm) and only a small effect on water conductivity. The pH shift was <1 unit and in compliance with pharmacopoeial requirements. Acetate and formate levels were determined by ion chromatography to be well below 0.1 ppm.
Table 3: TOC, conductivity, pH, and acetate/formate values for water extracts of tubing

Nonvolatile Residues (NVR): Table 4 lists total nonvolatile residues from the extracted tubing samples determined by oven-drying the extracts in preweighed crucibles. We found no detectable residues in the DI water extracts above the limit of quantification (LOQ) of 0.1 mg. Ethanol (100%), representing an extremely aggressive solvent for typical bioprocessing fluids, gave a mean value of 7,553 mg. Further analyses of these nonvolatile materials are described below.
Table 4: NVR results for water and ethanol extracts* of tubing

Identity of Tubing Extractables: Identification of individual extractable compounds in single-use component extracts is an important part of the qualification process. Using model solvents under exaggerated conditions and a range of analytical methods, it is possible to build up a comprehensive extractables profile. Table 5 summarizes our major findings from these studies on flexible tubing.
Table 5: Identity of extractables from flexible tubing
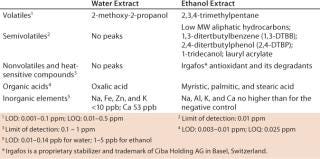
Results for DI water tubing extracts show very few organic compounds above the limits of detection (LOD). These data are consistent with the results in Tables 3 and 4, which show low levels of organic compounds and nonvolatile residues in DI water tubing extracts and minimal effects on solution conductivity and pH. With ethanol extracts, we identified additional compounds, as expected for this more aggressive solvent. Identified extractables were consistent with the tubing polymers and common additives of flexible thermoplastic tubing.
Ultraviolet (UV) spectroscopy absorbance values for the water extracts at 240 nm, 280 nm, and 350 nm were also very low and consistent with the above results. Metals analysis of ethanol extracts gave no different results from the negative control. For water extracts, only five elements (sodium, iron, zinc, potassium, and calcium) were >1 ppb but <10 ppb. Calcium was 53 ppb (0.053 ppm).
Polymeric Film Biocontainers
Polymeric film biocontainers and flexible tubing are single-use components that differ in their materials of composition and process application liquid-contact times. It is therefore important to perform separate extractables studies on biocontainers using appropriate test procedures and exaggerated conditions. Thus, a meaningful extractables profile can be established for these components, and their potential for leaching materials into pharmaceutical product streams can be assessed.
Biocontainer Properties and Extraction Conditions: We obtained Allegro biocontainers from Pall Life Sciences in East Hills, NY (www.pall.com). Table 6 shows the biocontainer properties and extraction conditions we used in our study. Fluids are often stored in biocontainers for extended periods of time, so we extended the contact time for extractables studies with the polymer film biocontainers to 30 days — compared with 72 hours (three days) for the flexible tubing studies. In addition, we used an elevated temperature of 40°C.
Table 6: Biocontainer properties and extraction conditions
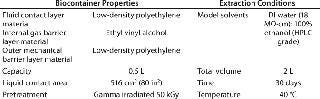
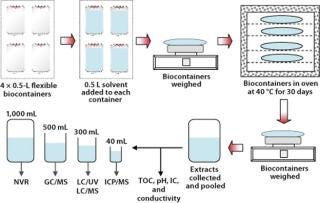
Figure 2 shows the extraction procedure diagrammatically. Each study involved a set of four 0.5-L biocontainers, giving a total extraction volume of 2 L. We extracted two sets and calculated the mean values for the results given below. And we used a PTFE bottle as a control.
TOC, Conductivity, pH, and Acetate/Formate: Table 7 presents the results of these analyses on water extracts from the biocontainers, which show low TOC levels and conductivity. The pH shift was <1 unit, in compliance with pharmacopoeia requirements. Formate was undetectable, and acetate was at a very low concentration of 0.2 ppm.
Table 7: TOC, conductivity, pH, and acetate/formate values for water extracts of biocontainers

Nonvolatile Residues (NVR): Table 8 lists total nonvolatile residues from the biocontainer extracts. Values were extremely low for water extracts (2.8 mg) despite the extended contact time of 30 days and elevated temperature of 40°C. Under the same exaggerated conditions, 100% ethanol (an aggressive model solvent for bioprocessing fluids) extracted a mean weight of 86 mg from four biocontainers.
Table 8: NVR results* for biocontainers

Identity of Biocontainer Extractables: Table 9 lists the major organic compounds and inorganic elements identified in water and ethanol extracts of the biocontainers. The results for water extracts show very few organic compounds above the LOD, and those identified were in the low-ppm or -ppb range. For ethanol extracts, the additional compounds identified were mostly oligomers of the base polymers or degradation products of the antioxidants used in those polymers. The finished film has been tested for biological safety during core validation of the biocontainer by the manufacturer/supplier (6).
Table 9: Identity of extractables from biocontainers
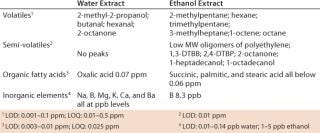
UV spectroscopy absorbance of both water and ethanol extracts at 240 nm, 280 nm and 350 nm were also very low and consistent with the above results. Metals analysis showed only boron in the ppb range (8.3 ppb) for ethanol extracts and boron, sodium, magnesium, potassium, calcium, and barium at low-ppb levels for water extracts. It is impossible to detail here all identified compounds because of space restrictions. However, the information is available on request.
Discussion
Our results on disposable tubing and biocontainers, together with those from the previously published study on single-use connectors and filters (1), demonstrate some important basic principles in process qualification for extractables and leachables. First, a relevant, comprehensive qualitative and quantitative profile of extractables can be obtained through a thorough understanding of single-use equipment; extraction conditions based on biopharmaceutical manufacturing process conditions; and analytical detection using advanced, validated techniques. With this approach, we generated a more detailed and relevant package of extractables data than is typically available from suppliers of single-use components. Such documentation can help users perform more accurate risk assessments for disposable-component qualification based on supplier-generated extractables data and assess potential leachables under drug product or process fluid-contact conditions. It may also, in some cases, minimize the need for additional process-specific extractables or leachables testing.
Second, the ability to identify organic and inorganic compounds at very low concentrations provides valuable information on the properties of tested single-use components. In addition to generating information on the chemical species extracted from unused components, these analytical data also help users determine the ability of their components to withstand worst-case process conditions.
In our studies, we used ion chromatography (with a detection limit down to 1 ppb) in aqueous extracts to assess levels of formate and acetate. Both compounds can be found in small quantities in most plastics and may come from raw materials — but they are also the smallest break-down products of larger molecules and thus can serve as strong indicators of chemical instability of a component, especially under extreme conditions. In the four widely different components we tested, the levels of formate and acetate were always below 1 ppm — and in most cases even below the LOD. Our data offer strong assurance of not only low formate and acetate leachables in product systems, but also good chemical resistance of the components even under exaggerated or worst-case process conditions.
Headspace analysis for volatiles by gas chromatography with mass spectrometry (GC/MS) provided valuable information on volatile organic molecules. These can come from a number of sources including monomers and oligomers, additives, residues from polymer treatment, residual solvents, and break-down products. For less volatile compounds, “standard” direct-injection GC/MS is also a powerful tool for identifying higher–boiling-point molecules, which may include oligomers and break-down products from antioxidants. Organic acids (e.g., stearic, myristic, and palmitic acids) are associated with the raw materials of various polymers. We identified them by GC/MS assay after derivatization to create suitable corresponding esters. For compounds that cannot be analyzed by GC, we used LC/UV/MS. It was particularly suitable for detecting nonvolatile molecules such as phenolic antioxidants. It is not suitable, however, for analysis of oligomers from some polymers such as polypropylene.
All those methods were used in our extractables studies for biocontainers, tubing, sterile connectors, and filter capsules. They provided important information on the organic molecules present and possible degradation products, and the results revealed compounds typically associated with the materials of construction. For aqueous extracts, our analyses showed extremely low levels, typically in the ppb range. Because most bioprocess systems use aqueous-phase processing, these results provide high assurance of low leachables from the four components tested.
Levels in ethanol extracts were higher, as expected, and we detected some additional compounds. Most ethanol extractables were <1 ppm, however, and none were above 15 ppm. Such low levels of extractables were present despite our use of an aggressive organic solvent (100% ethanol) and extreme test conditions to maximize extraction.
We obtained a similar pattern of results using inductively coupled plasma mass spectrometry (ICP/MS) analysis. This method for inorganic element characterization is particularly important for detecting metals from catalysts used in polymerization processes and from certain additives in polymers. Detection of undesirable heavy metals (e.g., lead) is also an important target for this analysis. We looked for ≥19 elements, and typically no more than six were above LOD — the most common being calcium, sodium, and potassium. No lead was found.
For nonvolatile residue (NVR) analysis, we saw significant differences in water and ethanol extracts. For example, the NVR value for water extracts from polymer film biocontainers was only 2.8 mg, but the ethanol extract was 30× higher. This was not an unexpected result in view of the solvent properties of 100% ethanol and the oleophilic or nonpolar nature of some extractables. That ethanol extract value was 90× less than that from tubing extracts. Comparing such very different products and test methods can be very misleading, however, and should be avoided. Process SOPs such as preflushing can radically reduce the quantity of extractables leaching into process fluids (leachables). This NVR example demonstrates the importance of developing separate extractables profiles for each component type using specific test procedures, then referencing the results for a total system package.
TOC, pH, and conductivity measurements can be performed only on water extracts. They were not only an important measure of potential effects on pharmaceutical products but also demonstrated compliance with GMP requirements. In all three assays, we found relatively small differences between component aqueous extracts and negative controls, results that are consistent with low levels of extractables shown by other methods.
Ongoing Work
Having now completed and published extractables studies on four single-use components — connectors, filters, tubing, and biocontainers — we can now begin building an extractables profile of a system containing all these major components. The results will help users make objective and more informative risk assessments for total system extractables.
Work is now in progress on a total extractables study of this four-component system, and the results will be compared with our composite profile obtained from individual component studies. This type of core validation data from suppliers can help users in component selection for their bioprocesses. It can also provide support documentation for process validation as well as, in some cases, possible reduction of the need for process-specific qualification of extractables and leachables.
1.) Ding, W, and J Martin. 2008. Implementing Single-Use Technology in Biopharmaceutical Manufacturing: An Approach to Extractables/Leachables Studies, Part One — Connectors and Filters. BioProcess Int. 6:34-42.
2.) Colton, R. 2007. Extractables and Leachables Subcommittee of the Bio-Process Systems Alliance. Recommendations for Extractables and Leachables Testing, Part 1: Introduction, Regulatory Issues, and Risk Assessment. BioProcess Int. 5:36-44.
3.) Colton, R. 2008. Extractables and Leachables Subcommittee of the Bio-Process Systems Alliance. Recommendations for Extractables and Leachables Testing, Part 2: Executing a Program. BioProcess Int. 6:44-52.
4.) Goldstein, A. 2006. Disposable Systems in Biopharm Production: Design, Compliance, and Validation Methods — A Case Study (Part 2). Amer. Pharm. Rev. 9:10-25.
5.) Goldstein, A. 2005. Methods and Considerations for Disposables Implementation: Design, Compliance, and Validation. BioProcess Int. 3:S20-S27.
6.) USTR2475Validation Guide: Allegro™ Biocontainers, Pall Life Sciences, East Hills.
You May Also Like