Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
July 1, 2010

IDBS provides a flexible data management solution that meets the diverse demands of biologic drug development based on our extensive experience working with global biopharmaceutical and contract manufacturing organizations. the IDBS Bioprocess data Management solution enables streamlined execution of process workflows, improved data access, sharing and reporting, and the process insight needed to successfully reduce the time and cost required to deliver new biologics to market.
Managing Diverse Data
Biopharmaceutical companies generate a vast amount of high-value data that is then locked in paper records, enterprise databases or Microsoft® office file formats. This makes it problematic to realize the full value of these important assets and maintain a holistic view of the processes being developed.
The IDBS Solution creates a fully electronic development history record across the development lifecycle, helping process engineers to develop robust, scalable and transferable bioprocesses. By integrating and optimizing workflows, the system enables more efficient process execution and reporting, and provides a basis for Quality by Design. Interactive reporting tools for monitoring process data, efficiency and quality provide the process insight required for troubleshooting and continuous improvement initiatives. The IDBS solution manages the flexibility required by development users, together with the more fixed nature of Good Manufacturing Practice (GMP) processes, in one integrated environment, ensuring the whole organization can gain insight and understanding from both the development and manufacturing lifecycles (Figure 1).
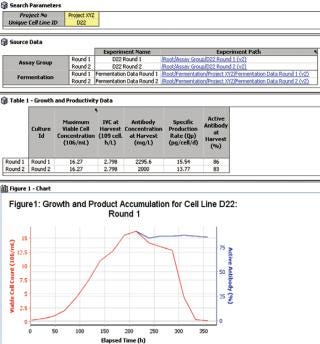
Figure 1: ()
Reduced QA Burden
Direct data entry templates and forms enable structured data entry in both GxP and non-GxP environments. Templates enable standardized recording of data, ranging from observations to instrument data files, allowing quality to be designed into the process execution and analysis work flows. Work flow templates can be used to capture batch records and deviations or to ensure that existing SOPs are adhered to throughout the development lifecycle. This reduces the GxP QA burden because errors are designed out of the work flows and deviations can be automatically highlighted through dashboards or email alerts, with the detailed context available for drill down.
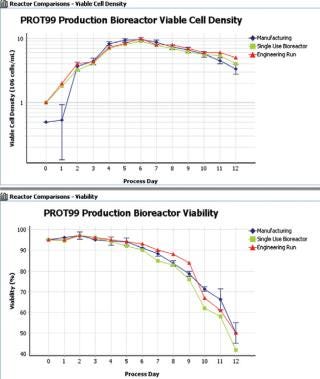
Figure 2: ()
Automated Report Generation
Information-rich reports can be simply created using drag-and-drop functionality, or specific report templates can be created to ensure consistency of reporting and comparability between results. Report generation times are proven to be dramatically reduced, enabling rapid delivery of development history, biological license applications and comparative analysis reports and campaign summaries.
Improved Data Visibility and Reuse
Powerful search capabilities provide enhanced visibility of all development, production and QA /QC data. All data is fully searchable, promoting re -use of high value knowledge and enabling better risk-based decisions and improved process insight. This ensures that there is a significant reduction in the “re -invention of the wheel” scenario that is a result of not being able to quickly locate past data and its associated context. the IDBS solution integrates process data from sources such as process data historians or chromatography controllers directly into the execution workflows, thus enabling real time process insight and expediting process investigations and decision making (Figure 3).
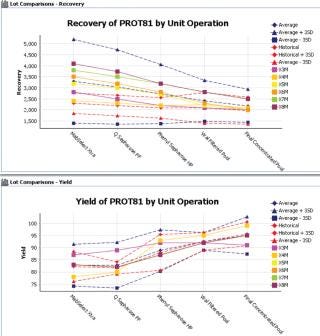
Figure 3: ()
Summary
The IDBS Bioprocess Data Management Solution provides one compliant portal for process development and biological production operations. By improving workflow execution, data and knowledge capture, analysis and accessibility, the IDBS Solution enables users to very simply answer who, what, when, how, where and why questions throughout the development lifecycle, helping to contract the development timeline. For the first time, scientific innovation, process execution, compliance and business performance can be seamlessly managed in one integrated solution.
About the Author
Author Details
Pietro Forgione is in biopharmaceutical business development at IDBS, [email protected], +44 7712 831 755, www.idbs.com.
You May Also Like