Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

The use of 0.1-µm rated sterilizing grade filters has been increasing in cell culture media filtration due to mycoplasma contamination concerns. Currently, no standards exist for conducting mycoplasma retention testing on these 0.1-µm labeled filters. A task force has been established at PDA to publish a technical report to standardize testing. Until such guidelines are published, conducting side-by-side tests under identical conditions is the only fair way to compare different 0.1-µm rated filters. Sartorius Stedim Biotech has conducted such tests on its Sartopore® 2 0.2/0.1 PES 10” cartridge filters at a third party lab using a modified ASTM F838-05 method to show A. Laidlawii removal at LRV = 7/cm2.
Such mycoplasma-retentive filters usually have tight final membrane layers, and a high-performance prefilter is usually required to optimize the filter train. SSB has recently launched a new line of PES prefilters: Sartoguard®. These Prefilters are available in double layer PES as well as triple layer configuration with depth filters such as GF upstream. a combination of Sartoguard® 0.1 nominal → Sartopore® 2 0.2/0.1 has been tested into two media applications: the most common soy-hydrolysate supplemented serum-free media and the strategically important fully chemically defined media. These tests were conducted on 47-mm disks with Pre → Final filters in series under 25 psig constant upstream pressure. Media were prepared from powder in accordance with manufacturer’s instructions.
For the serum-free soy hydrolysate supplemented media, all Sartoguard® filters performed very well with v∞ values > 6,000 L/m2. Sartoguard® GF was the best prefilter with v∞~18,000 L/m2, indicating flow-rate driven filtration. The number of 10” elements needed was also calculated for each media assuming 5,000 l batch size and two-hour filtration time. Sartoguard® GF/PES → Sartopore 2 0.1 were the best combinations overall, with 1-30” filter needed for each stage. A leading competitive combination required 2-30” filters for each stage (Figure 1).
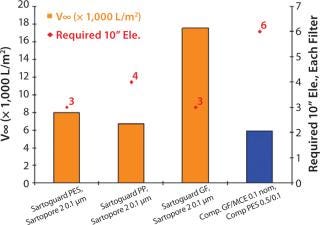
Figure 1: ()
The second media tested was a fully chemically defined media with high lipid content. As expected, this media was more difficult to filter. All combinations except Sartoguard® PES exhibited v∞ values > 1,000 L/m2 (~6× less than previous media), indicating throughput driven filtration. Sartoguard® GF was the lead prefilter candidate once again closely followed by Sartoguard® PP and the competitive combination with v8 ~1,000–1,400 L/m2. Number of 10” elements needed for 5,000 l batch size for Sartoguard® GF → Sartopore® 2 0.1 was 17 each followed by 18 each for Sartoguard® PP → Sartopore® 2 0.1, and 19 each for the leading competitive combination (Figure 2).
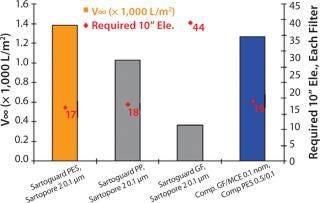
Figure 2: ()
In conclusion, Sartoguard® 0.1-µm nominal prefilters showed outstanding performance in two different types of cell culture media filtration applications in upstream processing. An application-specific approach of employing different versions of Sartoguard® prefilters proved to be successful as well. We would like to acknowledge SAFC Biosciences for providing different catalogued formulations for this testing.
About the Author
Author Details
Chris Sullivan is an applications specialist, and Mandar Dixit is head of product management for filtration technologies in North America, Sartorius Stedim Biotech; [email protected].
You May Also Like