Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
July 1, 2010

Chromatographic resins for a new purification process are initially selected based on prior experience and knowledge of the feedstock and the target compound. As the process scales larger, and hopefully through therapeutic commercialization, developers seek opportunities to maximize throughput, remove impurities, and reduce cost.
Responding to the dramatic increase in batch titers over the past decade, products such as the Toyopearl GigaCap series of resins (first introduced in 2007) were developed to meet the titer challenge with their high capacities and faster elution kinetics. Not surprisingly, process developers are taking advantage of these developments to improve their process platform by replacing traditional purification media with high dynamic binding capacity (DBC) resins for the capture step.
While this approach is making a positive impact in the industry, the combination of higher protein titers of feedstocks, higher DBCs of the capture resins, and a 60–70% reduction in target elution volume requires process developers to pay attention to protein solubility and precipitation. The interplay between protein titer, elution volume, and protein solubility is balanced by the process developer to take full advantage of the new high DBC capture resins. This leaves the intermediate purification steps as the next focus for process improvement.
DBC Driven or Selectivity Driven?
Protein purity, and thus chromatographic selectivity and resolution, becomes a stronger driver than binding capacity in intermediate purification steps. In some cases it makes sense to simply change the current particle size to a smaller one to increase resolution, while maintaining selectivity. Although this capability may not be available from other vendors, Tosoh Bioscience offers resins of the same chemical composition in particle sizes ranging from coarse (100 µm) to smaller (20 µm) grade materials.
Not Six Sigmas, But Six Particle Sizes
This article focuses on two chromatographic ligands: SuperQ and SP. These two products are available in six base bead particle sizes of 100, 65, 35, 30, 20, and 10 µm. The 100, 65, and 35 micron resins are sold under the Toyopearl trade name. The 30, 20, and 10-µm materials are sold under the TSK-GEL trade name. The only difference is that the smaller particle size TSK- GEL products are prepared with slightly more crosslinking in the polymer backbone to impart more rigidity to the resin so as to withstand the higher intrinsic backpressures they generate. Figure 1 shows the particle size continuity for our SuperQ and SP resins. As the particle size increases, the resin’s pressure flow properties favor being used as the capture step. As the particle size decreases more theoretical plates are generated resulting in improved resolution for more difficult separations such as n – 1, n, n + 1 oligonucleotides.

Figure 1: ()
Preserved Selectivity
Because the selectivity of the resins, and thus the elution order of the feedstream components, remains the same, the same buffer system, gradient composition, and so forth can be applied to each particle size separation after allowing for changes in column size and aspect ratio. Figure 2 shows the preserved selectivity for the SuperQ resin set. Figure 3 shows similar data for the SP resin set. (Please note that the 10-µm particle sizes are available only in our prepacked line of TSK-GEL HPLC columns.)
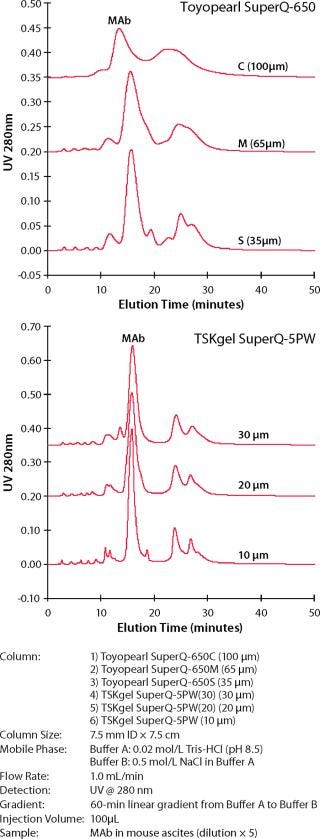
Figure 2: ()
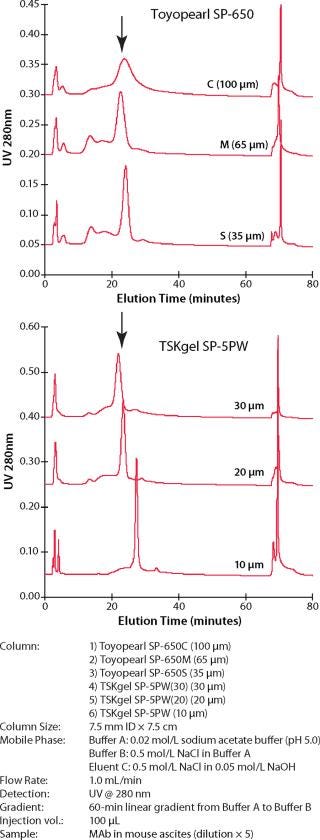
Figure 3: ()
In both figures, the column sizes are 7.5 mm ID×7.5 cm. The buffer and gradient conditions for the six-particle resin set is kept the same for each experiment. Similar data are available that show how selectivity is preserved for DEAE and CM ion-exchange products and phenyl hydrophobic interaction resins.
Conclusion
Five ligands are available as preserved selectivity resin sets: SuperQ, SP, DEAE, CM, and Phenyl. A separation developed on one particle size can be applied to the other particle sizes in the set. A separation developed during R&D on an analytical TSK-GEL HPLC column using one of the five ligands noted can be scaled for process purification through the use of the larger bulk particles.
About the Author
Author Details
Al Jackewitz is the biopurification market manager at Tosoh Bioscience LLC, 3604 Horizon Drive, Suite 100, King of Prussia, PA 19406; 484-805-1219; [email protected]; www.tosohbioscience.com.
You May Also Like