Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

It is important to ensure that testing services meet applicable regulatory standards necessary to support investigational new drug (IND) applications, the clinical development life cycle, and commercialization. However, monitoring and — when possible — anticipating the ever-changing regulatory landscape is a challenge. Yet, the failure to do so can negatively affect both the cost and time needed to move a product from development through to commercialization.
This article describes BioReliance’s position concerning anticipated regulatory changes over the next three to five years. These opinions are based on BioReliance’s experience providing testing services to 600+ clients and regulatory guidance published by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and international Conference on Harmonization (iCH). These references include
21 CFR Parts 210 and 211, Current Good Manufacturing Practices (GMP) in Manufacturing, Processing, Packing, or Holding of Drugs and Finished Pharmaceuticals
21 CFR Part 58, Good Laboratory Practice (GLP) for Non-Clinical Laboratories Studies
European Union (EU) Guide to GMP
European Clinical Trials Directive 2001/20/EC
UK Medicines and Healthcare products Regulatory Agency (MHRA) GLP Regulations
International Conference on Harmonization (ICH) E6, Good Clinical Practice
Clinical Laboratory Improvement Amendments (CLIA) (Title 42-Public Health, Part 493, Laboratory Requirements).
Table 1: BioReliance’s “future vision” for the regulatory testing landscape
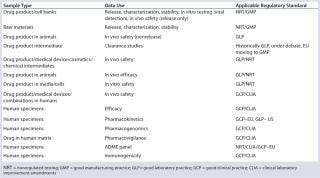
Table 1: BioReliance’s “future vision” for the regulatory testing landscape ()
The nonregulated testing (NRT) standards should be applied to ancillary testing supporting drug products. This type of testing is the “pure” development work required to prepare a drug candidate for IND submission and narrow processing requirements for drugs in the clinical life cycle.
For each of the other regulatory standards (GLP, GMP, GCP, and CLIA), BioReliance has defined minimum requirements. Clients must compare their own regulatory minimum requirements with BioReliance’s interpretation to ensure alignment or a clear understanding of the differences found. Any discordant views should be discussed and addressed in a quality agreement between BioReliance and the client.
In presenting this “future vision,” BioReliance sees some variation with current client/industry interpretations. The top three anticipated differences center around our application of GCP, GLP, and GMP standards.
The first involves defining GCP regulatory standards for testing. BioReliance established its GCP requirements based upon CLIA requirements. This interpretation features protocols describing testing and final reports capturing testing results. All testing performed includes positive and negative controls.
The second difference concerns the use of GLP regulatory standards for testing. Often GLP standards are applied to testing done in support of early phase clinical trials. In these circumstances, the scope of the GLP Regulations, 21 CFR Part 58.1 clearly indicates GLPs are for conducting nonclinical studies. Also the concept of having a test article applied to a test system for a GLP study is not fulfilled. Often, industry confuses the definitions of a test article versus a sample. These terms have different definitions within the regulations. Laboratory instruments should not be considered as GLP test systems. Without meeting these two basic definitions of test article and test system, testing should not be performed using GLP regulatory standards.
The final difference involves the use of GMP testing earlier in clinical development. IND and early clinical-phase testing should depend on compendial and BioReliance’s in-house GMP validated methods. This approach allows the GMP standard to be used while defraying the cost for development of product specific methods. The GMP standard in these circumstances should be performed for both drug product and associated raw materials. Current regulatory interpretation includes cell banks as raw materials, and therefore, these should also be tested to the GMP standard.
BioReliance uses this “future vision” interpretation to assist clients by providing feedback and advising on test selection and regulatory risks. This “future vision” allows BioReliance to best position clients not only to meet today’s regulatory challenges, but also to reduce regulatory risks, and client development costs.
About the Author
Author Details
Ron Hinkel is director of quality systems at BioReliance, 14920 Broschart Road, Rockville, MD 20850; 1-301-610-2687; [email protected]; www.bioreliance.com.Patricia P Rossman is president of Globiox, Inc. PO Box 91297, Austin, TX 78709-1297; 1-512-850-0467; [email protected]; www.globiox.com. Martin Wisher, PhD, is senior director of quality assurance and regulatory affairs at BioRelianceInnovation Park, Hillfoots Road, Stirling, Scotland FK9 4NFE; 44-141-579-3319; [email protected]; www.bioreliance.com.
You May Also Like