Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2011

Disposable cell culture bags are used in conjunction with rocker platforms in an array of cultivation applications (insect and mammalian cell lines, virus, protein expression). Rocking platforms provide the dynamic motion and oxygen transfer for cell culture environments. Disposable culture bags are designed to allow for a large air-liquid surface for oxygen transfer, easy access for sampling/filling, and a sterile environment. Venting issues have been reported in the field during rocking of disposable cell culture bags. Therefore, venting and cell growth testing were performed for optimized Charter Medical Clear-Pak® cell culture bags. Product Optimization
An optimal cell culture design was prototyped with modifications. These changes were chosen to address venting in the cell culture bag and incorporated into standard Charter Clear-Pak® 10-L cell culture bags (5-L working volume). Bags were gamma irradiated. Venting Experiment
A venting study was first executed to gage suitability of the modifications prior to cell cultivation. Two 10-L bags with the proposed modifications were tested: 1 bag with 2″ (5cm) tubing from chamber to filter and 1 bag with 8″ (20cm) tubing from chamber to filter. As in Figure 1, the venting study showed acceptable results for the Charter cell culture bags. Acceptable results were defined to be no clogging of filter or back-pressure spikes (>0.6 psi or >4.1 kPa) during rocker operation.
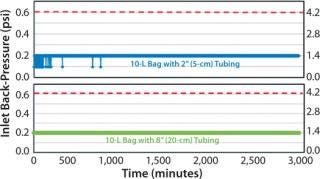
Chinese Hamster Ovary Cell Experiment
A cell growth experiment was executed to monitor viable cell count and cell viability (%) over seven days. Initial (t = 0) viable cell count and cell viability for the cell culture bag were 0.38 × 106 and 99.4%, respectively. The bag was tested for seven days and sampled daily for viable cell count and% viability. Nutrients and pH were monitored from daily sampling, but no feedback controls were used; therefore, no adjustments to the cell culture were made during the seven days.
As in Figure 2, the cell growth gave showed acceptable performance of the Charter cell culture bag design. Acceptable performance was defined by the high viable cell count after seven days and high cell viability for the duration of the test. The drop in cell viability at seven days (160 hours) is to be expected based on depletion of cell nutrients in the media and slowing of cell growth. Back pressure was also monitored and was equal to 0.1 psi during the seven days.
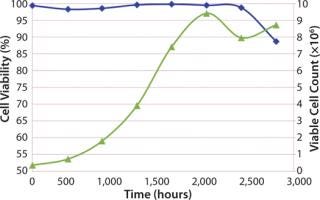
Conclusions
Data collected from this testing provide evidence that the Charter Clear-Pak® cell culture bags produced optimal performance for the cultivation of non-adherent CHO animal cells. Full protocols and processes can be found in an application note available at www.chartermedical.com.
About the Author
Author Details
Jared Ragone is new product development engineer at Charter Medical, Ltd., 3948 Westpoint Blvd., Winston-Salem, NC 27103; 1-336-714-4272.
You May Also Like