Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2013

The need for a rapid turn-around in quantity (and the loss of technicians in many laboratories) has created a trend toward single-use vessels. Their advantages include no time lost in cleaning or sterilization (especially for bench-scale, autoclavable systems), no chance of inhibition or cross-contamination due to poor cleaning, and no need for cleaning and sterilisation facilities in a crowded laboratory.
For production-scale steel bioreactors, fully automated clean-in-place (CIP) and sterilization-in-place (SIP) has been possible for decades. By contrast, bench-scale systems required considerable downtime for disassembly, cleaning, reassembly, autoclaving, and cooling before use — with no guarantee that each step was carried out correctly and reproducibly. An innovation from INFORS HT brings the advantages of CIP/SIP to bench-scale bioreactors, offering a true alternative to single-use systems for microbial applications.

The LabCIP provides user-configurable recipes for cleaning, sterilization, and rinsing processes. It can be left to perform these tasks overnight so that vessels are ready to use the next day. For high-throughput fermentation with organisms such as Escherichia coli, that can double the productivity of each bioreactor unit. The system is reliable and reproducible, and it involves little input beyond priming the reagent bottles and pressing “go.” Despite all this functionality, the LabCIP module’s footprint is smaller than an A4 sheet of paper.

The effectiveness of sterilization with sodium hydroxide is high, even at a low temperature of 25 °C (Table 1). A typical sterilization procedure using a LabCIP is carried out at 60 °C, which speeds up the sterilization process dramatically. Sterility testing against a panel of highly resistant test organisms (E. coli, Pichia pastoris, and Geobacillus stearothermophilus spores) showed no contamination after six days of incubation following the CIP/SIP process.
Table 1:
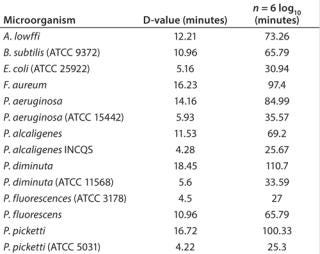
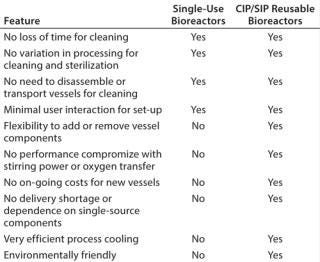
Direct comparison of single use systems and the self-cleaning and self-sterilizing bioreactor with LabCIP reveals the advantages of the new system (Table 2). Advances in automation for cleaning and sterilization now provide the best of all possibilities: customizable, high-performance bioreactors with no downtime related to manual cleaning and sterilization.
Table 2:
About the Author
Author Details
Dr. Tony Allman is product manager fermentation at INFORS HT, Rittergasse 27, CH-4103 Bottmingen/Basel, Switzerland, 41-61-425-7700, fax 41-61-425-7701; [email protected], www.infors-ht.com.
1.) Mazzola, PG. 2006. Chemical Resistance of the Gram-Negative Bacteria to Different Sanitizers in a Water Purification System. BMC Infect. Dis. 6:131.
You May Also Like