Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
January 26, 2018
Sponsored by GE HealthCare Technologies
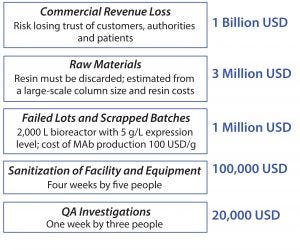
Figure 1: The extreme cost of a bioburden incident
Protein A has been a fantastic tool for the antibody industry. It is without doubt the most well-established purification technique in monoclonal antibody (MAb) manufacturing today due to a few key success factors. Protein A is the result of the long evolution of Staphylococcus aureus that developed a defense system against antibodies. Protein A exists on the cell wall of about 9% of S. aureus strains and immobilizes IgGs. When there is an immune response in the body, the bacterium defends itself by disabling IgG molecules, preventing them from attacking the cells. With this natural origin, it is hard to engineer an alternative with superior selectivity.
Despite protein A’s natural origins, the bioindustry has worked to make it more useful in industrial manufacturing by improving its alkaline stability. We have even played with the structure of protein A to increase the amount of binding domains. Throughout the generations of protein A resins, this engineering has made protein A a more effective tool at lower production costs.
Protein A is a proven technology. It is well understood by regulatory agencies, easy to move through clinical trials, and it has predictable operation. That said, there are still challenges that remain and opportunities to improve the technology. First, the protein A step is challenged by the increase of titers in bioreactors causing it to be the rate-limiting step in downstream purification. If we can increase the productivity of the protein A step, we can ultimately increase the productivity of an entire facility.
Second, protein A columns are generally too large. If you compare protein A to other types of resins, there is almost a one-to-two ratio in terms of capacity. That means that there is a limited window of operation for prepacked columns. The result is a mismatch between downstream operations and difficult choices to be made between whether you cycle a column (causing the process to take longer) or install a larger column (which involves additional maintenance and cost).
Third, protein A columns are the weakest link in downstream purification with respect to bioburden. Protein A columns are loaded with cell culture supernatant, creating a beneficial environment for bacterial growth, but the column is subsequently cleaned with the weakest clean in place (CIP) solution in downstream purification. Today, most protein A resins are compatible with concentrations of sodium hydroxide (NaOH) about 10× lower than subsequent steps, resulting in a much lower level of assurance against bioburden contaminations.
Finally, regulatory agencies are more interested in the issue of bioburden than ever before. They are asking whether biologics producers understand sources of bioburden in their processes. They want to see evidence that controls and analytical methods are in place to identify bioburden. Even in cases where bioburden hasn’t been an issue, if you start testing for it on a regular basis and find it, you have to do something about it.
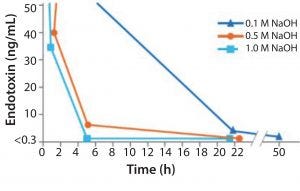
Figure 2: Increasing NaOH concentration shows a more effective endoxin inactivation
The reality of bioburden is everything from annoying to extraordinarily expensive (Figure 1). Even if a bioburden incident results only in a process deviation and investigation, most experts estimate the cost to be about 20,000 USD to investigate, perform a root cause analysis, and close the deviation. If you proceed through this escalating scale of cost, a bioburden incident could result in sanitization requirements for equipment or an entire facility, and result in failed lots of material. There are cases in which large-scale operations have had to replace the resin in a protein A column to recover from a bioburden incident. And, considering the cost, if this were to lead to an FDA warning letter or the shutdown of a facility, it could result in losses on the order of billions of dollars in reputational and commercial loss for a product on the market.
NaOH has been shown to be the most simple and effective cleaning and sanitization agent we have today (Figure 2). As the concentration of NaOH increases, it becomes increasingly effective at deactivating endotoxin and different types of both Gram-negative and Gram-positive bacteria.
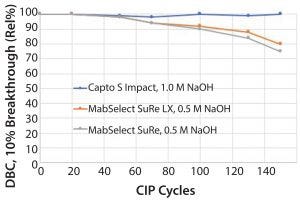
Figure 3: Capto S ImpAct, an ion-exchange resin, is fully stable in 1.0 M NaOH, but MabSelect SuRe or MabSelect SuRe LX media lose binding capacity with repeated exposure to
NaOH.
“Alkali-stabilized” protein A resins are still less resistant to NaOH than their counterparts in the rest of the purification suite. For example, Capto S ImpAct, an ion-exchange resin, is fully stable in 1.0 M NaOH, but MabSelect SuRe or MabSelect SuRe LX media lose binding capacity with repeated exposure to NaOH (Figure 3). This decrease in capacity occurs for essentially all protein A resins on the market today. That, of course, affects the process of economy.
At GE Healthcare, we have been thinking about these issues for several years. We have taken into account customer feedback and interviews conducted between 2011 and 2014. In 2014, we started a research project to identify concepts to address these issues, and in 2015 we started developing a next-generation protein A resin designed to address productivity and bioburden.
Starting with bioburden, the main goal has been to develop new ways that further engineer protein A to increase its alkaline stability. It has been 12 years since the commercialization of MabSelect SuRe resin, and technology has advanced since then. We went back to the drawing board to look for the weak points and determine what tends to be sensitive to NaOH. We considered what we can do to change those sites and increase stability without affecting the affinity of the ligand for IgG. The key to our work was using Biacore, which is a surface plasmon resonance technique. Using Biacore allowed us to evaluate ligands without having to synthesize resin. We could work at the molecular level and screen ligands using a high-throughput methodology.
We used the information gained from a series of experiments to create new libraries of ligands and make combinatorial changes in future designs. We screened more than 400 different constructs using this technique. As a result, we designed a monomer we thought was significantly more stable than what MabSelect SuRe is today.
We also looked at what would happen if we made various lengths of this new monomer, and we looked at the effect of the ligand length on various beads codeveloped at the same time. This is one of the first times we have (at least for an affinity resin) codeveloped the bead with the ligand to match the two materials to each other. If you think about how beads work, the larger the pores, the less diffusional resistance for IgG to get into pores, but there also will be less surface area. You have an optimization exercise here in which you are trying to create as much surface area as possible and immobilize as much ligand as possible, and also bind as much IgG as possible, while also trying to prevent a steric hindrance.
A bead with very small pores has a larger surface area but also more diffusional resistance. A bead with large pores reduces this resistance but may be softer and/or have less surface area. The project team worked to optimize the ligand design and bead design so that we have a ligand design that provides high capacity as well as a bead design that is well-matched to that construction.
The result of this work is the development of a new product we call MabSelect PrismA, the next generation of the MabSelect family. It is a new bead, and a new ligand. It is designed to solve the challenges described earlier: to resolve bottlenecks in production, and to have exceptional alkaline stability that allows for more stringent cleaning and better control of bioburden.
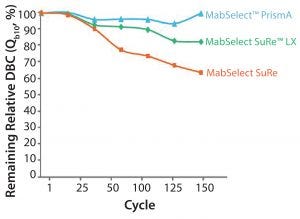
Figure 4: Cleaning with 0.5 M NaOH, 15 min contact time per cycle
Alkaline Stability: As shown in Figure 4, typically when cleaning with 0.5 M NaOH, you pay a penalty in terms of loss of resin capacity. The figure shows a comparison of MabSelect PrismA, MabSelect SuRe LX, and MabSelect SuRe. You can see we have maintained more than 95% of the dynamic binding capacity (DBC) with MabSelect PrismA, even over 150 cycles with 0.5 M NaOH. If you increase the NaOH concentration to 1.0 M, you can achieve even more stringent cleaning. This might be desirable if you want to align your downstream purification suite so that all of your resins are cleaned with the same CIP solution (e.g., 1.0 M NaOH).
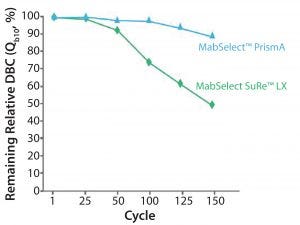
Figure 5: Cleaning with 1.0 M NaOH, 15 min contact time per cycle
Figure 5 shows a comparison of MabSelect PrismA resin with MabSelect SuRe LX resin. After over 150 cycles, MabSelect PrismA loses about 10% of its DBC whereas MabSelect SuRe loses approximately 50% of its DBC. MabSelect PrismA is significantly more stable than either MabSelect SuRe or SuRe LX. This increase in NaOH stability can be used both for increased cleaning, which can reduce batch-to-batch carryover, as well as to control bioburden outbreaks on the protein A column.
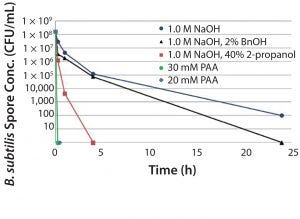
Figure 6: Reduction of Bacillus subtilis spores in 50% MabSelect SuRe™
Over the past few years, we have also developed a technique to recover from a spore-forming bioburden incident using an oxidizing agent called peracetic acid. This work has been completed for MabSelect SuRe (Figure 6). This recommendation will be extended to cover MabSelect PrismA as well. It can be a preventative or remedial measure to ensure that spore-forming bacteria don’t take hold on the protein A step.
Security of Supply Chain and In-House Bioburden Control: We have done considerable work around security of supply for MabSelect PrismA resin as well as work in bioburden control in our own manufacturing facilities. The agarose used to make the base matrix and the ligand itself are without doubt the two most critical raw materials, and we have validated in dual sources of both for MabSelect PrismA. For the ligand, GE is going to manufacture approximately 50% of the ligand supply, and a contract manufacturer who will use the same process will manufacture the remaining supply. This has been an additional investment on GE’s part to ensure we have full control of ligand manufacturing and to work with our contract manufacturer to make sure they have capacity to meet our ligand needs moving forward.
In our facilities, we have worked to make sure we are doing everything necessary to minimize bioburden in our products. We have a very good track record in this space, and have been operating in a scenario well within specifications for many years. In 2016 we felt it was time to make some updates to those specifications, and therefore we issued a change control notification announcing the reduction in bioburden specification from <100 cfu/mL down to <20 cfu/mL and the addition of a specification for endotoxin. Those two changes are the result of many years of in-house tests proving we have stayed well within specification. This doesn’t represent a change for end-users in the sense of what you are receiving from us because we have been well below these for many years, but it does give an added level of assurance that we will never release anything that is outside of the new specifications.
Since 2010, GE Healthcare has produced hundreds of lots of MabSelect SuRe. Our absolute, most common value for bioburden is zero. More than 94% of the lots released have no detectable bioburden. Over the same period of time, we have not measured a bioburden value higher than 5 cfu/mL. We have worked hard with our operators and manufacturing operations to help them understand the importance of hygiene on their operations and the impact it can have on our customers. We feel very proud of this result.
We recognized a couple years ago that protein A is a relatively susceptible step in downstream purification with respect to bioburden contamination. We also know that protein A in general has the potential to become rate limiting as titers increase. MabSelect PrismA is designed to address these issues by enabling the use of 1.0 M NaOH for cleaning and sanitization, offering significantly improved binding capacity.
Learn more: 
Jonathan Royce is Global Business Leader for Chromatography Resins at GE Healthcare Life Sciences.
You May Also Like