Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
October 22, 2019

Photo 1: TideXCell bioreactor platform from Esco Aster (Photo courtesy of Esco Aster)
This year’s BioProcess International Conference and Exhibition, held 9–12 September 2019, hosted nearly 200 exhibitors showcasing technical innovations for the biopharmaceutical industry. This year’s conference sessions also included daily technical workshops detailing supplier solutions and technologies. Below are some notable technologies featured that demonstrate the industry’s dedication to finding new ways to help manufacturers shorten time to market. Most systems offer the benefits of single use, integration, process control, economies of scale, automation, and closed-system processing.
Upstream Production
The exhibit floor had only a few booths from bioreactor suppliers, and most were familiar systems in single-use design and pilot and laboratory scales. Newer systems offered improved integration with software platforms and an increase in sensor monitoring. And there was a notable increase from last year in the number of services providers offering production of cell and gene therapies.
Bioreactor Systems: Esco Aster has developed a platform for producing adherent bone-marrow–derived and adipose-derived stem cells for allogeneic cell therapies. At the conference, the company showed its TideXcell 2-L bioreactor system, which includes a control and incubation unit that contains a matrix vessel with the company’s BioNOC II carriers. This system contains a matrix vessel and TideXcell mixing unit for holding mixing bags or vessels of culture media. The platform can be connected to the company’s harvest and feed tanks for perfusion and continuous culture (Photo 1 and on the cover).
During the week before the conference, ABEC announced that it had increased the volume of its single-use custom single run (CSR) bioreactor to 6,000 L. President and CEO Scott Pickering said, “In applications where single-use systems are preferred, . . . customers can now achieve even greater economies of scale.”
Rapid Sequencing: Highlighted during a conference presentation, the MinION portable device by Oxford NanoPore Technologies enables rapid DNA and RNA sequencing. Each flow cell generates up to 30 Gb of DNA sequence data or 7–12 million reads of RNA. The device streams data in real time and can be plugged into a PC or laptop.
Bioprinting: Adam Feinberg from Carnegie Mellon University presented on scaffold fabrication using three-dimensional bioprinting. His team has developed Freeform Reversible Embedding of Suspended Hydrogels (FRESH) technology to build gel-like scaffolds. Using seeded cells on those scaffolds, the researchers can produce soft-cell encapsulated materials and tissues such as in coronary arteries and other functional parts of a heart. When the printing process is finished, the hydrogel scaffold can be washed away.
Tissue Development: During a preconference symposium, Stuart Jacobson of the Advanced Regenerative Medicine Institute (ARMI) and DEKA Integrated System Corp presented the ARMI BioFab USA Tissue Foundry Integration pilot-project integrated process for developing tissues. The design is a closed, modular system with single-use components and includes programmable logic controllers, automated control, process monitoring, and data logging. The system so far comprises four modules: Module 0 consists of five cryovials in which cells are thawed automatically, then extracted. Module 1 is cell culture, using up to four vessels and with control of vital parameters such as pH, dissolved oxygen, temperature as well as monitoring of agitation and cell count.
Module 2 performs cell harvest and wash. Different module options for cell processing are under consideration. Module 3 enables tissue assembly and maturation. The system’s development is an ongoing collaboration project with biopharmaceutical researchers and manufacturers. Jacobson said that the long-term goal is to develop a Foundry 1.0 system as the first step toward an integrated, automated, closed, turn-key system for manufacturing tissue-engineered medical products (TEMPs).
Downstream Processing and Filling
This year, the exhibition focused primarily on streamlining downstream processes. A few suppliers offered systems to eliminate and/or replace bottleneck problems at the centrifugation and depth filtration steps. Newer downstream technologies also have been designed with small footprints and to incorporate more automation and disposable components than before.

Photo 2: Emphaze anion-exchange filter from 3M (www.3M.com)
Chromatography: Sartorius Stedim Biotech presented several technologies at its booth. Among them were a “PAT ready” single-use Biostat STR stirred-tank bioreactor and a rapid cycling chromatography (RCC) system for monoclonal antibody capture. The bioreactor is fully scalable and can be used for high-density, microcarrier, and perfusion applications. The system is designed for use with the company’s single-use bags (Flexsafe STR), which include single-use sensors for measuring multiple parameters. The RCC system is designed to reduce time to market by enabling high productivity. It comprises the “convecdiff” membrane, a range of scalable devices, and a supporting skid.
JSR Life Sciences presented its Amsphere A3 protein A chromatography resin and Chromasette chromatography platform. The resin consists of a highly cross-lined bead structure to provide high dynamic binding capacity (DBC) at high flow rates. The company reports that DBC to be 50% higher than DBCs of market-standard resins at two- to six-minute residence times. In 2016 the company acquired the Chromasette technology from SPF Technologies. Representing the first modular chromatography platform, Chromasette devices have stackable, disposable cassettes for scale-up. Each cassette has an internal scaffold that supports resin beads to reduce resin compression.
Prometic featured Evolve bioprocess columns for pilot and commercial-scale chromatography. The columns come with a kit, which contains tools, connections, and spare accessories for “ready-to-use” implementation. The columns have a unique “semidisposable” design that enables operators to change out all wetted parts and use “refresh kits.”
3M featured Emphaze anion-exchange (AEX) chromatography hybrid purifiers (Photo 2). The technology includes all-synthetic materials (polymers, fine-fiber nonwoven media, and bioburden-reduction membranes). It provides flow-through chromatographic separation of contaminants to reduce DNA, host-cell proteins, and endotoxins. When used with the company’s Zeta Plus depth filter, the purifier enables centrifuge-free direct harvest clarification.
Xavier de Mollerat du Jeu, director of product management of cell biology at Thermo Fisher Scientific, presented his talk on bridging the gap from development to commercial production. He introduced the audience to his company’s Rotea counterflow centrifugation instrument. Scheduled for availability in 2020, the technology is a closed cell-processing system for cell therapy manufacturing. Offering high recovery, the system is designed to process up to 20 L of starting volume continuously and deliver concentrate volumes as small as 5-mL output with up to 200 million cells/mL. A single-use kit enables scale-up from research to commercial scale.
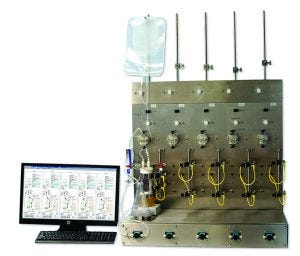
Photo 3: PendoTECH’s five-station tangential-flow filtration system (photo courtesy of PendoTECH)
Filtration: PendoTECH featured a five-station tangential-flow filtration (TFF) screening system (Photo 3) and sensors (page 10). The TFF system enables users to perform parallel experiments and interact with the system through a PC-based graphics user interface (GUI). Six built-in programmable recipes can be used for batch control. Pressure sensors can be cleaned and reused, and the company’s throttle valve controls back pressure.
Repligen presented its new Krosflo TFDF (tangential-flow depth filtration) integrated clarification technology (Photo 4) and ProConnex TFDF Flow Paths assemblies. The TFDF technology takes processes from harvest to retentate — eliminating the centrifugation step or reducing depth-filter requirements — in a closed, single-use, automated system. The Krosflo instrument includes integrated software and an automated process control logic system.

Photo 4: Repligen’s Krosflo tangential-flow depth-filtration process system (photo by Maribel Rios)
ErtelAlsop featured Microcap Pro single-use diafiltration capsules for batch filtration. The capsules include a disposable microfiltration technology for large scales. The capsules can be used for pilot and production-scale processing or when users want to convert from reusable to single-use processing.
Resins: Purolite Life Sciences presented its jetting technology resins made of copolymer and agarose beads (Praesto range). The proprietary technology produces resins with specific pore and porosity structures for purifying monoclonal antibodies and other complex molecules.
Buffer Delivery: MilliporeSigma featured its Biocontinuum buffer delivery platform as a step toward enabling continuous and intensified biomanufacturing. It is a configurable, connected system comprising buffer concentrates, buffer dilution, and single-use assemblies for buffer preparation and management of large volumes of buffers. The platform is designed to eliminate the need for manual buffer preparation.

Photo 5: Vanrx SA25 robotic filling workcell
(photo courtesy of Vanrx)
Robotic Filling System: A Vanrx SA25 workcell is a closed, aseptic, robotic filling and handling machine. Photo 5 shows such a system in use at Singota Solutions, a contract development and manufacturing organization (CDMO) that built its first aseptic filling capacity for vials, syringes, and cartridges on the filling machine. At the conference, senior marketing manager Eric Petz told me, “The key attribute leading to speed to market is a standard design that allows construction, qualification and validations to happen in shorter time frames than is typical in the industry. Over the past two years, customers have averaged 7.5 months from purchase order to factory acceptance test (FAT) and 1.75 months from FAT to the completion of a site acceptance test (SAT), including shipping time.”
Analysis
Several suppliers offered automated analytical solutions for replacing tedious manual tasks and in some systems, enabling the capture of real-time process data.
Sphere Fluidics featured its Cyto-Mine single-cell analysis system for antibody discovery and cell line development. The instrument offers a high-throughput method of encapsulating cells in picodroplets of growth media, letting them grow in a compartmentalized state, and trapping secreted molecules (antibodies) for selective screening of single cells.
Beckman Coulter presented its Biomek i5 automated workstation for liquid handling. The system (and the i7 version, not shown at the conference) has an open platform with a grid-based deck and performs multichannel pipetting tasks in 0.5–5,000 μL volumes. The instrument includes process management and scheduling software for “walk-away” operation. Other models demonstrated at the conference were the company’s ViCell Blu and Metathex liquid-handling systems.
Some conference presenters described the capabilities of the digital polymerase chain reaction (dPCR) technology, a more precise method than traditional PCR. At the exhibit, Bio-Rad featured its Droplet Digital PCR (ddPCR) system, which performs dPCR based on water–oil droplets. The droplets separate template DNA molecules and serve the same function as individual tubes or wells in a plate in which PCR reactions traditionally take place.
Securecell presented its Numera automated sampling system for online analytics. The instrument automatically draws biomass-containing samples from bioreactors and then processes, stores, and automatically transfers those samples to directly coupled analytical devices. The company also featured its Lucullus process information management system (PIMS) for use with the Numera system.
Flownamics also featured an automated online sampling system. Seg-Flow instruments sample eight vessels or process streams and deliver those samples to four analyzers of fraction collections. The system can integrate with several commonly used bioprocess analyzers and software systems.
![]()
Photo 6: Beacon optofluidic platform from Berkeley Lights (photo courtesy of Berkeley Lights)
Some conference presenters discussed their companies’ use of the Beacon optofluidic platform from Berkeley Lights (Photo 6). This digital cell analysis system processes thousands of cells rapidly for applications such as cell selection, gene editing, and antibody discovery. OptoSelect chips move cells in parallel while they are cultured, assayed, and measured. Integrated software captures and displays results, which also can be shown as digital graphics.
In his conference presentation, Synthace chief scientific officer Markus Gershater introduced “computer-aided biology” and the need to “connect the digital world with the physical world, powered by artificial intelligence (AI) and automation.” The company’s Antha laboratory software platform links laboratory automated systems to data integration by controlling and coordinating multiple laboratory devices. “Typical laboratory analysis planning takes a lot of time,” he said. “We developed software tools to help carry out laboratory automation and flexibly perform a large range of protocols. If you need to change parameters, the system automatically reprograms those protocols for you. For example, if you want to increase the number of dilution steps, the software allows you to do that easily and will make changes along your entire experiment.”
Show Us Your Technology
The BPI conference exhibition included many more technologies that could not be mentioned because of space limitations here. Most suppliers are designing their systems to fit the need to reduce time to market by implementing automated, single-use, integrated, and closed systems. Additional technologies are presented in the following pages. If your company’s technology isn’t listed in this report, please send me an invitation to stop by your booth during next year’s conference — or better, let BPI editors know that you’d like to work with us in publishing your content.
Maribel Rios is managing editor at BioProcess International; [email protected].
You May Also Like