Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

LFB Transgenic Goat
Antithrombin alfa is a recombinant human antithrombin developed as an anticoagulant treatment for people with hereditary antithrombin deficiency who are undergoing surgical or childbirth procedures (1). Marketed under the ATryn brand name by LFB SA (Les Ulis, France), antithrombin alfa was approved for use in adults by the US Food and Drug Administration (FDA) in February 2009 (2).
Antithrombin alfa is expressed in the milk of transgenic goats and purified through a multistep downstream process encompassing both filtration and chromatography. The source material (transgenic goat milk) naturally contains a microbiological load. To manage that, the collection process is controlled carefully, resulting in a historical average bioburden of 1,930 CFU/mL (n = 61) for pooled source material. Such a level is well below the prepasteurization standard for US grade-A cow’s milk of ≤100,000 CFU/mL (3). LFB’s ATryn clarification and purification process is designed to clear bacterial load rapidly from source material. Both bacterial load and endotoxins are monitored throughout the process to confirm rapid clearance of the native flora and demonstrate process control.
In addition to the starting bacterial load, there is some potential for a fungal load to be present in the source material. Even with a well-controlled collection process for goat milk, it is unavoidable to have occasional fungal load present in the animals’ environment. Thus, LFB monitors total yeast and mold throughout collection and processing, with both shown to be cleared quickly and early on in the purification process. However, it was not known whether potentially toxic by-products of fungal growth (mycotoxins) might accompany the presence of mold because those are not easily measured or monitored in process streams.
Mycotoxins are toxic secondary metabolites produced by some filamentous fungi (molds), including Aspergillus, Penicillium, and Fusarium species (4). These toxins are typically a concern in storage of cereal grains because they are produced under specific conditions of temperature and pH, with the availability of simple carbohydrates for active fungal growth — often present in grain-storage applications (4). Animals that consume contaminated feeds can carry detectable levels of mycotoxins (primarily aflatoxin M1) in their milk. Accordingly, the FDA has set an aflatoxin action limit of 0.5 ppb in milk and a maximum level in the feedstock of lactating dairy animals of 20 ppb (5, 6). Vendors providing goat feed to LFB are selected with those limits taken into consideration, and all goat feed is tested for aflatoxins to ensure mycotoxin safety and eliminate diet as a potential source of mycotoxin exposure.
We previously examined the potential for fungal growth and/or mycotoxin generation in transgenic rabbit milk, which is the source material for LFB’s FDA-approved Sevenfact (recombinant coagulation factor VIIa) (7). The study demonstrated robust safety levels over time after spiking with a quantitative amount of commercially available fungal standard. We did not repeat the same type of milk-growth study for our antithrombin alfa source material because of general milk properties (pH, temperature, and carbohydrate profile) that are unfavorable to mold propagation.
For the follow-on research reported herein, our sole aim was to improve our understanding of mycotoxin risk for antithrombin alfa drug substance by determining the mycotoxin clearance capability of its purification process. For these clearance evaluations, we spiked relevant commercially available mycotoxins into process fractions from a scaled-down antithrombin alfa purification process representation to determine its ability to remove mycotoxins if they were present in the source material. Then we calculated the clearance potential for each process step and summed the results to determine a total mycotoxin clearance level for the whole process. We used those results to calculate potential risk to patients through a theoretical dosing exercise, by which we estimated a safety factor in excess of published mammalian toxicity levels.
With hundreds of possible mycotoxins in the environment, it is impossible to evaluate each one individually (8). For these evaluations, therefore, we have classified them by general physiochemical properties and then applied a bracketing approach by evaluating only the worst-case examples for which commercial mycotoxin standards were available. To support that strategy, we developed a high-performance liquid chromatography (HPLC) method that could resolve 10 different commercially available mycotoxin standards easily.
We chose rugulosin (among the most hydrophobic) and deoxynivalenol (DON, the most hydrophilic) mycotoxins for our process clearance studies because they bracketed all the other standards in hydrophobicity assay evaluations. Physical size was considered to be a constant because nearly all mycotoxins have a molecular weight of <1,000 g/mol, which is at least 50× smaller than the antithrombin molecule (4). We also considered ionic interactions of mycotoxins to be constant because most such toxins are not charged molecules, and they generally are only slightly soluble in water. Therefore, we considered their variability in hydrophobicity to be the largest differentiator for the purposes of our study, using standard mycotoxins that would bracket the entire observed hydrophobicity range.
Materials and Methods
Mycotoxin Preparation: DON from MilliporeSigma and rugulosin from Abcam were dissolved in ultrapure dimethyl sulfoxide (DMSO) from VWR to a working concentration of 2.5–3.5 mg/mL and stored at –20 °C until use. We used the mycotoxin stock solutions as both HPLC assay standards and process-spiking materials. As explained above, DON and rugulosin were chosen to represent a full range of hydrophobicity based on retention-time results of 10 different mycotoxins evaluated in a carbon-18 (18C) HPLC assay.
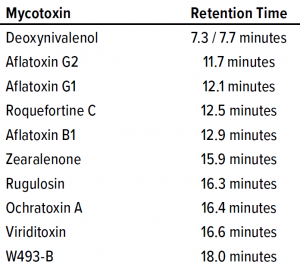
Table 1: Retention times of different mycotoxins on the carbon-18 (18C) column.
HPLC Mycotoxin Assay: Mycotoxins were separated on a 4.6 mm × 150 mm 5-µm Gemini C18 column from Phenomenex, with mobile phases containing 5 mM ammonium acetate with 1% acetic acid. Methanol was added at 10% and 97% for mobile phases A and B, respectively. We adapted a linear gradient method from a Phenomenex technical bulletin (9), with detection at both 260-nm and 280-nm absorbance. Ten mycotoxins were evaluated during assay development: rugulosin, viriditoxin, deoxynivalenol, aflatoxins (B1, G1, and G2), ochratoxin A, zearaleone, roquefortine C, and W493-B. Table 1 lists their relative retention times. We generated standard curves individually for each mycotoxin used in our process clearance study.
Sample Extraction Procedure: Before analysis, samples of each spiked process fraction and resultant process step outputs were collected and stored at –80 °C. To each 200-µL sample, we added 600 µL of DMSO. Samples were vortexed and passed through a 10-kDa molecular weight cutoff (MWCO) Microsep Advance polyethersulfone (PES) filtration membrane from Pall to remove residual protein. We loaded the filtrates directly onto a 18C column without dilution. Extraction efficiency was evaluated but not considered to be a factor in our study calculations because clearance was determined by direct comparison of process step inputs and outputs, with extraction efficiency assumed to affect each equivalently.
Process Scale-Down: Each process step was scaled down to minimize fraction volumes while maintaining process comparability. When possible, process steps were performed at the same scale as previous process robustness studies. Each process step was scaled down ~200×–2,000× (Table 2) depending on the available laboratory-scale filtration and chromatography formats. We evaluated five downstream process steps for mycotoxin clearance ability: combined microfiltration and affinity chromatography, ultrafiltration/diafiltration (UF/DF), anion-exchange chromatography (AEC), and hydrophobic-interaction chromatography (HIC).
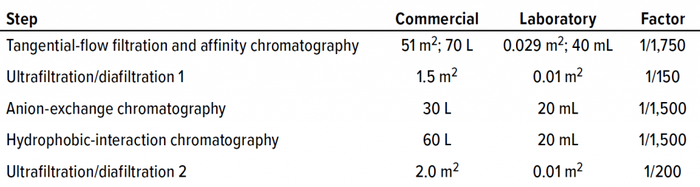
Table 2: Mycotoxin clearance process scale-down
Results
Process Clearance Study: To demonstrate proportionality between concentration and peak area and to determine the minimum detectable amount of each mycotoxin, we began by preparing a series of standards in DMSO for rugulosin and DON. Rugulosin eluted from the 18C column as a single peak with maximum response at 260 nm (Figure 1). DON eluted as a doublet with peaks at 7.3 minutes and 7.7 minutes. Maximum absorbance was at 260 nm for the 7.3-minute peak and 280 nm for the 7.7-minute peak, and we summed the two for DON quantitation purposes: DON = (7.3-minute peak area at 260 nm) + (7.7-minute peak area at 280 nm).
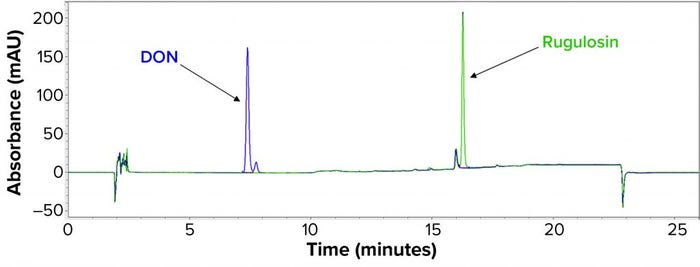
Figure 1: Carbon-18 (18C) chromatogram showing relative retentions of rugulosin and deoxynivalenol (DON).
As Figure 2 shows, both mycotoxin standard curves were highly linear, with significant differences in absorptivity between rugulosin and DON (with absorption of the latter about 16-fold lower). Limits of detection (LoDs) were determined to be 0.1 µg/mL for rugulosin and 0.5 µg/mL for DON.
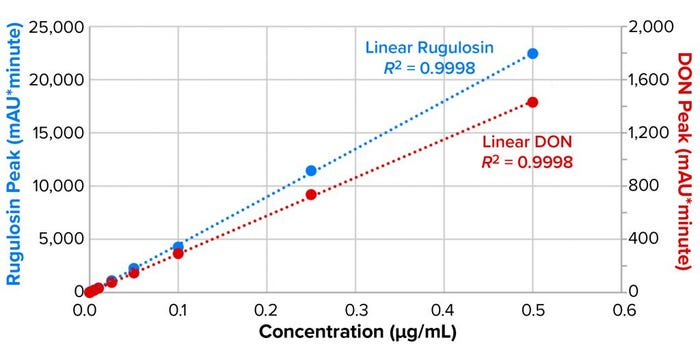
Figure 2: Limits of detection (LoD) and coefficient of determination curve fitting for
rugulosin and deoxynivalenol (DON).
Next, we spiked combined samples of rugulosin and DON mycotoxin standards into the input of each step in the ATryn downstream process. With those spiked inputs, we collected the output of each process step according to normal process operations. Samples of each input and output were extracted with DMSO, and the proteins were removed with a 10-kDa membrane. Filtrates then were loaded directly onto the 18C column for determination of the resulting mycotoxin peak areas. Output samples that were below the LoD were loaded at larger injection volumes to increase the sensitivity of our technique by fivefold. We determined the step clearances by comparing mycotoxin peak areas multiplied by the sample volumes between input and output fractions, then reported the results as logarithmic reduction values (LRVs) (Table 3).
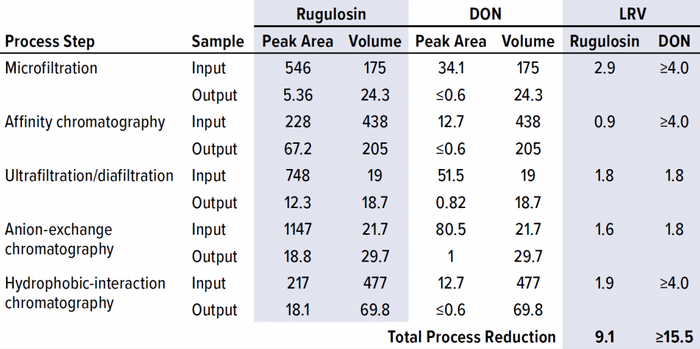
Table 3: Mycotoxin clearance from process-stream material at each step in the downstream process; LRV = log-reduction value, DON = deoxynivalenol.
All process samples contained an interfering peak at 16.3 minutes that could not be resolved from the rugulosin peak at the same retention. We confirmed that interfering peak to be present in unspiked process samples that had been subjected to the same extraction process. Through further investigation, we determined that the peak had been extracted by DMSO from the 10-kDa PES membrane used to remove the protein before 18C analysis. We considered alternative mycotoxin extraction methods to eliminate this DMSO/PES chemical incompatibility issue. However, no other solvent could extract the majority of the mycotoxins consistently from the different sample matrices from the ATryn process.
Instead, we developed a background-subtraction technique by relating the 16.3-minute extract peak to another PES extract peak at 17.0 minutes that was isolated spatially in the chromatograms. Thus, we determined that the 16.3-minute extract peak was consistently 40% of the 17.0-minute peak in unspiked investigational extracts, regardless of the sample matrix. So we calculated the rugulosin peak area in our spiked samples by subtracting 38% of the 17.0-minute peak from the total peak area at 16.3 minutes. The remainder provided a reliable estimation of the rugulosin peak area in the presence of technique-based background peaks.
Patient Safety Calculation: For the sake of patient safety, we chose a conservative starting point by assuming that 1 g of mycotoxin were present in the source material of a drug product (DP) batch. The maximum dose for a 75-kg patient would be 8.2 vials of the 1,750-IU DP format, which represents 0.68% of the total batch. Using the minimum clearance determined in this study (9.1 log10 for rugulosin) and the maximum dose proportion to a DP batch, a total of 5.4 pg of mycotoxin could be delivered to a patient using that estimate. Hence, by conservatively adopting a median lethal dose (LD50) of 40 mg/kg — 49–70 mg/kg intraperitoneal in mice for DON (10) and 44 mg/kg intraperitoneal in rats for rugulosin (11) — based on the mycotoxins studied, we calculated the worst-case dose to be at least 100 billion times lower than the published mammalian LD50 values.
Discussion
LFB initiated this study to estimate the potential for mycotoxin exposure in patients using the ATryn (antithrombin alfa) clotting factor. To bracket the full range of potential mycotoxins from different mold sources, we chose two test standards (rugulosin and DON) to represent the range of associated physiochemical properties. Each mycotoxin was spiked into material representative of individual purification unit-operation inputs for determination of the process-step clearances. Solvent-extracted samples were quantitated using a 18C RP-HPLC assay.
Each mycotoxin studied was cleared effectively by the multistep purification process, with a minimum cumulative clearance of 9.1 LRV. Based on the DON clearance data, three of the five process steps proved to be highly effective in clearing mycotoxins. As expected, the two size-based process steps, microfiltration and UF/DF, efficiently removed mycotoxin because of the large size differential between antithrombin alfa and the mycotoxin molecules. The next-most effective process step was affinity chromatography, in which the mycotoxins were not bound to the resin’s functional groups and thus washed away before product elution.
Based on these study results, we calculated patient risk using source material and DP batch sizes, with a maximum dose for a 75-kg patient and the process clearance values reported herein. Under the worst-case assumptions described above, the concentration of potential mycotoxins was at least 100 billion times lower than published mammalian LD50 values for both DON and rugulosin. Note that these estimates are based on source material containing a theoretically extreme amount of mycotoxin, which would require substantial mold growth in the goats’ milk. Conditions favoring such growth do not occur in normal production operations: Source material is frozen quickly and then processed immediately after thawing and pooling, which are performed at controlled chilled temperatures.
References
1 ATryn Package Insert. LFB: Les Ulis, France, 2009, revised December 2013; http://www.atryn.com/pdf/ATrynPI_1750-525_Combo_December_2013.pdf.
2 ATryn. US Food and Drug Administration: Rockville, MD, 5 March 2018; https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/atryn.
3 Grade “A” Pasteurized Milk Ordinance. US Food and Drug Administration: Rockville, MD, 2019; https://www.fda.gov/media/140394/download.
4 Janik E, et al. Molecular Aspects of Mycotoxins: A Serious Problem for Human Health. Int. J. Mol. Sci. 21, 2020: 8187; https://dx.doi.org/10.3390%2Fijms21218187.
5 Center for Food Safety and Applied Nutrition. FDA Compliance Policy Guide Section 527.400: Whole Milk, Lowfat Milk, Skim Milk – Aflatoxin M1. US Food and Drug Administration: Rockville, MD, November 2005; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-527400-whole-milk-lowfat-milk-skim-milk-aflatoxin-m1.
6 CVM/ORA. FDA Compliance Policy Guide Section 683.100: Action Levels for Aflatoxins in Animal Food. US Food and Drug Administration: Rockville, MD, March 2019; https://www.fda.gov/media/121202/download.
7 Allard G, et al. Risk Determination of Potential Mycotoxin Exposure to Patients. BioProcess Int. 19(10) 2021: 46–50; https://bioprocessintl.com/analytical/downstream-validation/mycotoxin-clearance-risk-determination-for-exposure-to-patients-testing-recombinant-human-factor-vii-from-transgenic-rabbits.
8 Brase S, et al. Chemistry and Biology of Mycotoxins and Related Fungal Metabolites. Chem. Rev. 109(9) 2009: 3903–3990; https://doi.org/10.1021/cr050001f.
9 TN-1119: Mycotoxins and Other Fungal/Bacterial Metabolites from Several Food Matrices by LC/MS/MS. Phenomenex: Torrance, CA; https://phenomenex.blob.core.windows.net/documents/c8b71f29-fc33-478b-ac0a-3950ee59d092.pdf.
10 Sobrova P, et al. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 3(3) 2010: 94–99; https://doi.org/10.2478/v10102-010-0019-x.
11 Ueno Y, et al. Toxicological Approaches to (+) Rugulosin, an Anthraquinoid Mycotoxin of Penicillium rugulosum Thom. Japan. J. Exper. Med. 41(3) 1971: 177–188; https://www.jstage.jst.go.jp/article/jts1976/5/4/5_4_295/_article/-char/ja.
Greg Allard is a principal scientist in process and analytical development, and corresponding author Sean Evans is senior vice president of manufacturing and development at LFB USA in Framingham, MA; 1-508-494-8773; [email protected]. Both authors codesigned the studies and cowrote the manuscript; Allard performed the experiments. ATryn and Sevenfact are registered trademarks of LFB SA. Microsep is a registered trademark of Pall Corporation.
You May Also Like