Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Generic validation is conceivable only through a thorough understanding of the parameters affecting the performance of a process step. In this paper, we provide a detailed example demonstrating the robustness of a virus filtration step. As a first step towards the establishment of a generic validation package for a monoclonal antibody, the robustness of clearance of PP7 across the ViroSart CPV filter was evaluated by changing several critical operational parameters using a simple one-off experimental design. Two different validation approaches were used during this study: the classical validation approach and the “run-and-spike” approach. This first screening experiment, combined with data accumulated for several other products, provides valuable insights for the future development and validation of robust virus filtration steps.
Virus filtration has become a standard feature of modern biomolecule purification processes. Indeed, this step is generally capable of providing robust clearances of small viruses, such as parvoviruses, through a size exclusion mechanism. Thus, virus filtration is considered to be “orthogonal” to all the other purification process steps generally evaluated for virus clearance, such as chromatography and inactivation (1). The implementation of such virus retentive filters in a manufacturing setting is relatively simple, as they do not require specific equipment and are generally available as disposable units. However, virus filtration membranes are prone to rapid fouling. Such fouling can generally be attributed to the characteristics of the material loaded: the protein concentration, the presence of aggregates and the presence of residual DNA are often cited among the possible culprits (2,3,4,5,6,7,8,9). Virus filtration membranes are also thought to be sensitive to other operating parameters (feed pressure, pH or conductivity), although no clear evidence of such influences has been published. Premature membrane fouling results in dramatic losses of throughput, translating into significantly higher manufacturing costs — in a monoclonal antibody purification process, virus filtration is the second most expensive process step, right after Protein A affinity chromatography. Therefore, to maximize throughput, virus filtration steps are placed at the end of the purification process, where the purity of the material applied to the membrane is the highest.
During the validation studies aimed at demonstrating effective viral clearance under the actual filtration conditions, a few more difficulties are encountered: despite the tremendous efforts made to use representative scale-down models of the filtration process, through the strict control of operating conditions (composition of buffers, feed pressure, pH, conductivity, product concentration range, etc.), the validation experiments introduce unavoidable sources of non-representation: First, the virus spikes introduce a very large quantity of virus particles in the starting material. Such an occurrence is highly unlikely at the end of a purification process. In addition, the virus stocks used during spiking are themselves derived from cell cultures. Depending on the cell culture conditions and the purification process selected, the purity of virus stocks may vary from Contract Research Organization (CRO) to CRO, but also from lot to lot within a single CRO. Finally, the dilution buffer used for the resuspension of the purified virus stock may also contain components, which affect filter performance (such as calf serum). The level of impurities introduced in the feed material by the virus spike may directly impact the outcome of the validation study itself. Membrane fouling and overloading lead to rapid flux decay, which may in turn, for some filters, result in premature virus breakthrough into the filtrate (2,4,5,6,7,8,9). Considering the above, two future major challenges can be readily identified for the validation and implementation of virus filtration steps:
A manufacturing challenge: The constraints on the purification processes will continue to increase in the coming years, due to the preponderance of monoclonal antibodies in the development pipeline. Monoclonal antibodies are generally administered in high doses. The titre improvements in cell culture and the increasing batch size will result in higher batch volumes and product concentrations. Increasing throughput — and decreasing manufacturing cost of goods — will therefore become a prime concern for the implementation of virus filtration steps.
A regulatory challenge: In the context of quality by design and risk-based quality management, regulatory agencies will be placing a greater emphasis on process knowledge and on the evaluation of the robustness of all process steps. For virus filtration steps, robustness of clearance will not only be demonstrated by the fact that high clearances can be achieved with different viruses, but rather by the comprehensive understanding of how modifications of the critical operating parameters may affect viral clearance.
In this paper, we assess the robustness of a virus filtration step by screening a wide range of operating conditions, using two different virus spiking approaches. We also show how the leveraging of results obtained during previous studies on similar products may also be considered as an important source of information to assess the robustness of a virus filtration step.
Materials and Methods
Materials
Virus Spike: Bacteriophages have been used in the past as models for small (2,3,4,5,6,7,8,9) and large (3,10) mammalian viruses for the challenge of virus filtration devices. Small phages, such as PP7 used in this study (25 nm, non-enveloped, ssRNA, Leviviridae phage family), are approximately the same size as the small mammalian parvoviruses generally used during viral clearance validation experiments. They can usually be grown to high titres, are easily purified and safely handled. The PP7 stocks used in this study were prepared within the Virology Laboratories of Sartorius-Stedim Biotech (Goettingen, Germany). Initial virus spikes of about 7.7 Log10 are usually achievable. Considering a quantification/detection limit at 1–2 Log10 for the titration method, the expected dynamic range for the viral clearance was around 6 Log10, which was deemed to be sufficient for this evaluation. All the spiking experiments were also performed within the Virology Laboratories of Sartorius-Stedim Biotech.
Model Protein: The model protein was a therapeutic monoclonal antibody provided by Merck-Serono. The starting material was an intermediate fraction collected from the purification process. This fraction was aliquoted, and each aliquot was adjusted according to the experimental plan, conditioned and shipped to Sartorius–Stedim Biotech for the spiking experiments.
Parvovirus-Retentive Filters: The virus filter evaluated during this study was the Virosart® CPV from Sartorius, in the MiniSart configuration. Filters from two lots numbers were used in this study: lot number 0750173 R.20 Z.1/1 (lot A) and lot number 0750373 R.36 Z.1/1 (lot B).
Methods
Run-and-Spike Approach to Viral Clearance Validation: The run-and-spike approach has been recently proposed as a viable approach to viral clearance validation (4). In contrast with the standard spike-and-run approach, where the virus spike is added prior to running the filtration, in this case the virus spike is added after the total volume of starting material has been filtered, and virus clearance is evaluated at the end of the filtration. The development of this approach involves several different steps:
• Prior to validation, the filterability of representative material should be evaluated to determine the length of the “run” phase to be applied during the run-and-spike experiments. The volume of the “run” phase is determined on the basis of the maximum filterability observed, the trans-membrane flux measured during filtration, the batch size planned for manufacturing operations and the desired processing time. The volume of the “run “phase should correspond to the desired throughput in full-scale manufacturing conditions.
• During the validation, a standard spike-and-run experiment should be performed — with each virus — to define the spike ratio to be used during the validation study. The aim of these control experiments is not to maximize the throughput, but to find suitable spiking conditions to monitor the flux decay and the instantaneous clearance.
• The run-and-spike experiments are performed by first applying a volume of unspiked material as determined in the prevalidation filterability study. Then, a small volume of feed material is spiked with the spiking ratio defined from the spike-and-run experiments. The flux is monitored during both the run and spike phases. Several samples are collected during the spike phase to determine the instantaneous clearance and to compare it with results obtained during the spike-and-run experiments. The overall clearance in the filtrate pool is also determined.
The clearance factors obtained from the run-and-spike experiments may be considered as a worst-case scenario for the virus retention, as they correspond to the clearance of virus at the maximum throughput intended for the manufacturing operations; that is, when the risk of breakthrough is the greatest. Comparable clearance values between control and run-and-spike experiments would clearly indicate that the membrane “ageing” due to the run phase does not affect the performance of the membrane. Finally, the total validated throughput for the membrane is calculated by adding together the filtrate volumes collected during the run and spike phases.
Robustness Experiments: The screening experiments were performed following a “one-off” experimental design — one operating parameter was varied while all the others were maintained at their target point. The parameters considered during this study were pH, conductivity, concentration of the protein solution and feed pressure. The ranges investigated are shown in Table 1. Each experiment was performed in duplicate. In addition, the experimental matrix was repeated for two different filter lots following both the standard spiking method and the new run-and-spike method. In both cases, several grab samples were collected from the filtrate stream to characterize the eventual impact of flux decay on the retention performance of the filter. It is to be noted that, with this experimental design, one can easily assess the influence of each factor. However, potential interactions between multiple factors cannot be identified.
Table 1: Operational ranges defined for the investigation of the robustness of the viral filtration step
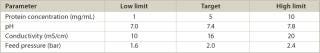
Table 1: Operational ranges defined for the investigation of the robustness of the viral filtration step ()
Results
In this robustness study, 32 different sets of operating conditions were tested: pH, conductivity, protein concentration and feed pressure were varied, one parameter at a time. Each set of operating conditions was tested for two different filter lots, in duplicate runs. For each experiment, the output parameters investigated were the PP7 phage clearance and the flux decay profile, as the latter is susceptible to virus retention, at least for some nanofilters (7,9). As mentioned earlier, the purity of the virus stocks used during the spiking experiments may affect the performance of the virus filters. To minimize the impact of the virus spike on the filter performance, several options may be considered. The first one is to increase the purity of the virus spikes. This may be achieved through the use of serum-free media during cell culture or the implementation of advanced virus purification techniques. The second option consists of evaluating alternative validation approaches. Several approaches, based on a better understanding of filtration processes, have been proposed recently, including the evaluation of the influence of the flux decay on viral retention, in relation to the pore plugging model.
In this study, we have decided to compare the “standard” spike-and-run approach with the run-and-spike approach described in the “Methods” section. Indeed, this new validation method presents several advantages. First, the “run” phase is usually much larger than the spike phase. Thus, it imposes a highly consistent throughput during the validation exercise, regardless of the type of virus used or the method of production of the virus. This is especially critical when establishing filtration endpoints for manufacturing operations: the validated throughput is thus clearly defined and is more representative than the one obtained from a classical spiking approach, owing to the elimination of the spike-related impurities during the run phase. Furthermore, by characterizing the influence of the flux decay on viral retention, the run-and-spike approach provides the additional assurance that the clearances observed during validation will be applicable to the manufacturing scale operations, provided that the appropriate flux control strategies are implemented. Last but not least, the validated throughputs are usually larger than for a standard spiking approach, which results in a significantly reduced sizing of the nanofilters in production. To complete the study, the entire experimental matrix was repeated with both validation approaches.
Flux Monitoring: The PP7 stocks used in this study exhibited high titres and low levels of impurities. Consequently, the addition of PP7 generally had a low impact on material filterability, even at the spike ratios used during the runs performed with the standard spiking approach. Figure 1 shows typical flow curves obtained during these experiments. For both spiking methods — standard and run-and-spike — the filterability target was reached for all experiments. For several experiments performed, the graphs suggest that there may be a slight influence of filter lot number on the filterability characteristics. This is especially evident with the standard spiking approach, where a clear partition of flux curves can be observed between lots A and B (see Figure 1). No such clear partitioning could be observed for the run-and-spike experiments. This may account for by the fact that, for the standard approach, the membrane is challenged from the beginning with the virus. This challenge will enhance eventual flux heterogeneities associated with lot-to-lot variability of the filter membrane. In the run-and-spike approach, the virus is spiked only at the end of the experiment, so most of the runs lie within the same trend, regardless of lot number.
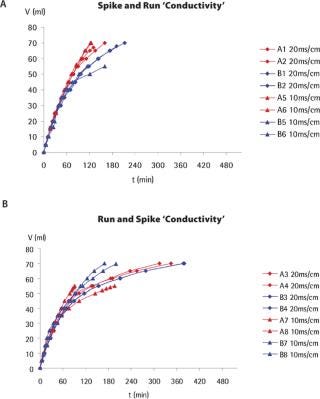
Figure 1: ()
During this study, large variations in flux (and therefore filtration times) could be observed between replicates and across filter lot numbers, but equivalent total throughputs were nevertheless achieved for all experiments performed at low or target protein concentrations. Taking into consideration this flux variability, no significant impact of conductivity, pH or operating pressure (results not shown) on the total throughput could be detected. By contrast, Figure 2 shows that product concentration had a strong influence on flux profile and total throughput for both spiking approaches. Little flux decay was observed at low protein concentrations (1 mg/mL). At a protein concentration of 5 mg/mL, flux decay was apparent but the target throughput was nevertheless reached. At higher protein concentrations, a much faster flux decay was observed, indicating that premature membrane fouling had occurred. In most cases, flux decays exceeding 90% were observed, and the experiments had to be interrupted with lower total throughputs. This behaviour was observed with both spiking approaches, clearly indicating that the membrane fouling is associated with the model protein fraction, and may not be attributed to an interaction of the virus spike with the concentrated product.
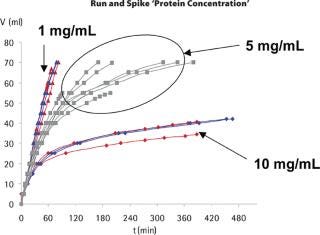
Figure 2: ()
Infectivity Testing: The grab samples collected along the filtration experiments were titrated for the presence of phage. In the case of the standard spiking approach, grab samples could be collected right from the start of the filtration experiments, so the instantaneous log reduction value (LRV) could be determined for a wide range of flux decays. For example, as shown in Figure 3a for the conductivity experiments, the LRV could be determined for flux decays between 10 and 60%. In the case of the run-and-spike approach, the “spike phase” is usually initiated with high flux decay values, so the range of flux decay for which instantaneous LRVs could be obtained is much more restricted for each experiment, but flux decay values can nevertheless be high (Figure 3b). As seen in the graphs, the instantaneous LRVs remained consistently high throughout the filtration experiments, and were not affected by level of the flux decay.
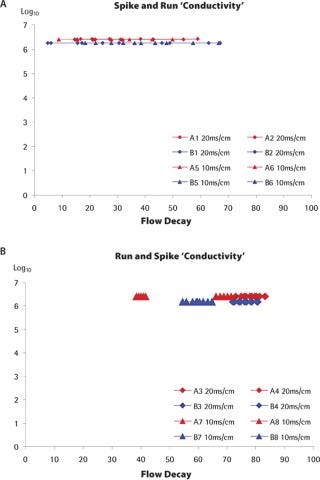
Figure 3: ()
The same trends were observed for spiking experiments performed at different pHs (results not shown). For one set of experiments evaluating the influence of pressure (filter lot A, standard spiking approach, low and high pressure), the LRVs recorded were somewhat variable. However, the fact that the duplicate experiments did not show consistent trends, that the same experiments performed with the other lot of filters did not show this variability and that the LRV was independent of the flux decay for all the run-and-spike experiments suggest that his variability was linked to a technical issue. In any case, even during those high variability runs, the LRVs remained above 5 Log10 throughout the duration of the filtration. In the previous section, it was shown that the protein concentration did have a significant influence on the flux characteristics of the filter membrane. The important flux decay observed was attributed to membrane fouling by the load material itself. Figure 4 shows the instantaneous LRVs determined from the titration of the grab samples collected during these experiments. Whatever the protein concentration, the virus clearance factors across experiments remained consistently high. The graphs also clearly show that, for all experiments performed, virus retention was not significantly affected by the level of flux decay. Even at flux decay values exceeding 90%, regardless of the spiking approach, no virus breakthrough could be observed, as would be expected from a membrane according to the pore plugging model. Finally, there was no impact of the spiking approach on the LRV levels. Therefore, the retention characteristics of the filter were not significantly affected by the “membrane ageing” imposed by the “run phase” performed prior to the spiking.
Figure 4: ()
Discussion
Conductivity, pH and operating pressure did not significantly affect the flux performance of the Virosart® CPV. Some slight differences in flux profiles were attributed to lot-to-lot filter variability, but this observation is consistent with previously published studies (7,9). The only process parameter that did significantly affect the flux characteristics of the filter was the protein concentration, probably due to membrane fouling. Nevertheless, using two different spiking approaches, it was demonstrated that the Virosart® CPV filter was capable of providing consistent retention of PP/7 phage from a monoclonal antibody solution over a wide range of operating conditions. These results are consistent with the current knowledge regarding virus retention mechanisms during nanofiltration:
Conductivity and pH: considering the structural properties of viruses and the tremendous pressures exerted by the packed nucleotide material on the capsids (equivalent to pressures up to 50 bar), it is highly unlikely that the shifts of conductivity and pH usually considered in a production process would significantly affect the size of the virus particles. Therefore, unless the membrane integrity is affected in some way, virus retention should not be significantly influenced by small shifts in pH or conductivity.
Generic validation is conceivable only through a detailed understanding of the parameters affecting the performance of a process step. In this paper, we provide a detailed example demonstrating the robustness of a virus filtration step. As a first step towards the establishment of a generic validation package for a monoclonal antibody, the robustness of clearance of PP7 across the ViroSart CPV filter was evaluated by changing several critical operational parameters using a simple one-off experimental design. Two different validation approaches were used during this study: the classical validation approach and the “run-and-spike” approach. This first screening experiment, combined with data accumulated for several other products, provides valuable insights for the future development and validation of robust virus filtration steps.
Operating Pressure: feed pressure is the driving force for filtration. The range evaluated in this study was relatively restrictive. In a manufacturing setting, the definition of the operating range for pressure will be driven by the specifications of the filter device, and the desire to operate the filter under conditions that will limit suite occupancy. Therefore, the specified range of operating pressure is expected to be minimal as well.
Under the conditions investigated in this study, the flux decay did not affect the virus-retentive properties of the Virosart® CPV, even under the high protein concentration experiments where significant membrane fouling was expected. This is in good agreement with previous publications that showed that — under the conditions tested up to this point — the Virosart® CPV does not seem to follow the pore-plugging model.
In fact, the experience accumulated at Merck-Serono has demonstrated that the Virosart® CPV, as well as other parvovirus-retentive filters, are capable of delivering consistent clearances (>4 LRVs) for small non-enveloped viruses over a wide range of operating conditions. The virus retention on the Virosart® CPV is indeed extremely robust: consistently high clearance factors (>4 LRV) for MVM were obtained with different types of molecules, from “lower molecular weight” hormones to Fc-fusion proteins or monoclonal antibodies, at product concentrations between 0.7 and 10 mg/mL, at various conductivities (2. In addition, up to this point, we have not been able to identify conditions where virus retention may be affected by the flux decay. Other parvovirus-retentive filters from different manufacturers also demonstrated consistent performance. In our experience, with different types of products, at concentrations between 0.4 and about 6 mg/mL, at operating pHs between 3.8 and 8.5, conductivities up to 70 mS/cm and operating temperatures of 2–8 °C and room temperature, consistently high retentions of MVM or other small viruses (about 25 nm) were obtained. However, for these filters, it is absolutely critical to pay attention to the operating mode of the filter membrane to avoid important overload conditions — as indicated by significant flux decays — which may lead to virus breakthrough.
For all virus filters, including the Virosart® CPV, the run-and-spike method as a new method for the validation of viral clearance represents an important improvement, as it provides a systematic approach for the evaluation of nanofilters based on a better understanding of retention mechanism. In addition, the “run phase” allows for the validation of consistent throughputs, regardless of virus stocks quality, which presents a great advantage for the definition of filtration endpoint in a manufacturing setting.
Conclusion
The “current” practice for the validation of viral clearance across nanofiltration membranes is to perform the spiking experiments under the (assumed) worst-case conditions, or at the very least under normal process conditions. This evaluation is performed with parvovirus as well as larger viruses in the panel to demonstrate the “robustness” of the clearance. However, current regulatory trends suggest that the emphasis will shift towards
Better Process Understanding: it is important to realize that virus filtration devices are not interchangeable and they do not behave the same way when challenged. Depending on the membrane design, some filters will adhere to the “pore plugging” model, whereas for others, the virus retention will remain unaffected by flux decay.
Design Space: The establishment of a “design space” that defines the operating ranges within which the safety (and efficacy) of the product is guaranteed. This represents a true test of the robustness of a viral filtration step, whereby the virus retention properties of a membrane will have to be evaluated under conditions mapping the proposed “design space.” This type of study may also lead to the establishment of modular validation packages in the case of platform processes.
The evaluation of the robustness of viral retention will combine information gathered from different sources: the literature, the information provided by the manufacturer concerning the operating mechanism of the filter and the stability of the membrane material, and the knowledge on the filter accumulated within the Company and documented within an internal virus filtration validation database for several products, under various pH and conductivity conditions. Some experimental work will remain unavoidable, but it may be possible to limit the scope of these additional experiments to the most challenging virus models. Alternatively, phages of equivalent size have been demonstrated to be excellent models for nanofitration evaluation, with the added advantages of ease of production and purification. Considering the mechanism of operation of filters, filter performance characteristics obtained with such parvovirus/phage studies could easily be extrapolated to all virus models of greater size, and therefore allow for a true and complete understanding of virus removal robustness.
1.) Carter, J, and H. Lutz. 2002. An Overview of Viral Filtration in Biopharmaceutical Manufacturing. Eur. J. Parenter. Sci. 7:72-78.
2.) Ireland, T. 2004. Viral Filtration of Plasma-Derived Human IgG: A Case Study Using Viresolve NFP. BioPharm Int. (online publication)..
3.) Aranha-Creado, H, J Peterson, and P. Huang. 1998. Clearance of Murine Leukaemia Virus from Monoclonal Antibody Solutions by a Hydrophilic PVDF Microporous Membrane Filter. Biologicals 26:167-172.
4.) Wu, Y. 2008. Validation of Adventitious Virus Removal by Virus Filtration – A Novel Procedure for Monoclonal Antibody Processes. BioProcess Int. 6:54-59.
5.) 2005. Virus filtration, Technical Report No. 41, PDA. Journal of Pharmaceutical Science and Technology Supplement Vol. 59.
6.) Zeechan, H. 2006. Protein Fouling of Virus Filtration Membranes: Effects of Membrane Orientation and Operating Conditions. Biotechnol. Prog. 22:1163-1169.
7.) Tarrach, K. 2007. Filtration: Vendor notes: The Effect of Flux Decay on a 20-nm Nanofilter for Virus Retention. BioPharm Int. (online publication)..
8.) Bolton, G. 2005. Normal Flow Filtration: Detection and Assessment of Endpoint in Bioprocessing. Biotechnol. Appl. Biochem. 42:133-142.
9.) Lute, S. 2007. Phage Passage After Extended Processing in Small-Virus-Retentive Filters. Biotechnol. Appl. Biochem. 47:141-151.
10.) Lute, S. 2004. Characterization of Coliphage PR772 and Evaluation of its use for Virus Filter Performance Testing. Appl. Environ. Microbiol. 70:4864-4871.
11.) Cordova, A. 2003. Osmotic Shock and the Strength of Viral Capsids. Biophys. J. 85:70-74.
You May Also Like