Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
ACMC Strategy Forum held in Washington, DC, on Sunday 28 January 2007, focused on two topics related to protein structure and function. First, analytical techniques used in the glycan analysis characterization included recent advances and correlations among the various tools. And second, current understanding glycosylation’s functional relevance to therapeutic proteins was discussed in the context of its effects on biological activity, pharmacokinetics, and Fc effector functions (for monoclonal antibodies, MAbs). Progress has been made in the field of glycobiology since this forum took place, and those updates can be found in articles referenced herein.
PRODUCT FOCUS: GLYCOPROTEINS
PROCESS FOCUS: MANUFACTURING
WHO SHOULD READ: PROCESS DEVELOPMENT, MANUFACTURING, ANALYTICAL AND QA/QC PERSONNEL
KEYWORDS: CARBOHYDRATES, MABS, ANALYTICAL METHODS, LOT RELEASE, STABILITY, CHARACTERIZATION
LEVEL: INTERMEDIATE
Human Interleukin 2 (IL-2) glycoprotein, a hormone that acts as a signaling molecule for the immune system, is used clinically as a cancer treatment. ()
Analysis and Controls
Analytical Tools: The tools most commonly used for glycan characterization include highperformance liquid chromatography (HPLC) and capillary electrophoresis (CE) based on detection modes that are either chromophores or fluorophores through derivatization. For HPLC, electrochemical detection systems are often used with underivatized carbohydrates. Mass spectrometry — whether matrix-assisted laser-desorption ionization (MALDI) or electrospray ionization (ESI) — is playing an increasing role here. MALDI time-offlight (MALDI-ToF) analysis gives a reasonably good glycoform profile rapidly enough to satisfy process analytical technology (PAT) principles, although getting actual percentage results takes days. Additionally, nuclear magnetic resonance (NMR) imaging can be used for glycans; however, it requires reasonably pure glycan structure in relatively large quantity.
How far we need to push the limits of analyses and controls depends on resolution, detection sensitivity, and speed. In principle, any given analytical methodology must find a balance between speed and resolution. It was pointed out in our discussions that when choosing a tool, it is advantageous to pick a method that does as little as possible to your sample. Another key factor in successful implementation of an analytical tool is operator training (the “human factor”). It is not unusual to see two different laboratory technicians come up with very different results, which often turns out to be caused by a slight difference in their techniques.
Another factor to consider is the accuracy of a method. Because of that consideration, we discussed the need for procedural reference standards or system suitability standards. Currently, no sufficient number of commercially available glycan standards are available with acceptable purity for generating a standard curve. Tina Morrison highlighted the fact that USP recently initiated an effort to establish such standards. Although external standards can provide useful calibration information among different methodologies, meeting participants recognized that for a given biological product, internal product standards or reference materials can serve the same purpose with the added benefit of product-specific information. Although the forum agreed that carbohydrate reference standards would be desirable, the consensus was that most analyses for lot release are based on relative quantitative measures (e.g., glycan profiling) rather than absolute quantitative measures. So the lack of external reference standards has not yet constituted a true limitation.
THE MORNING SESSION
The morning session’s goal was to develop a general understanding of the variety of analytical tools available for carbohydrate analysis: their limitations, strengths, and suitability for use in routine lot-release monitoring.
Presentations: The carbohydrate analysis plenary session was chaired by Rohin Mahtre (Biogen Idec) and Blair Fraser (FDA CDER):
“Glycoprofiling Strategies to Support Production of Recombinant Therapeutic Proteins,” by Shekar Ganesa (Genzyme)
“Future Trends in the Measurement of Biopharmaceutical Glycosylation,” by Daryl Fernandes (Ludger Ltd.)
“Human Glycoforms Produced by GlycoFi Engineered Yeast Strains,” by Andy Standheim (GlycoFi).
Key Workshop Questions in the morning workshop discussion panel included
What are the general capabilities and limitations of key analytical tools available in glycan characterization?
How far do we need to push the limits in complex glycan structures?
What are the general approaches used for characterizing glycoproteins (monosaccharide analysis, glycan profiling and identification, site occupancy)?
What key factors will differentiate a characterization technique from one used for routine lot release? What would the key characteristics/expectations be for an in-process or PAT application?
Attendees agreed that no single method or approach can provide a full picture of glycan structure. Thus, the need to use a panel of comprehensive and complementary tools is acknowledged. However, we generally agreed that analytical tools in use today are adequate despite some limitations in their speed and detection limits.
Characterization: To fully characterize a glycoprotein, general approaches focus on two of its aspects: carbohydrates and the protein itself.
Both monosaccharide analysis and glycan profiling are typically used to characterize the carbohydrates. Methods for looking at released carbohydrates are limited by the fact that it is impossible to get 100% release, and the group agreed that 85% is generally considered an acceptable release extent. Although oligosaccharide profiling provides a more comprehensive fingerprint of carbohydrate structures and relative distribution, gross monosaccharide analysis does not provide the same level of information (analogous to amino acid analysis of an antibody). However, monosaccharide analysis is useful in some cases, particularly when considering total composition of a protein related to O-link or specific terminal monosaccharides (e.g., terminal GlcNAc in the case of Genentech’s Lenercept p55 tumor necrosis factor receptor fusion protein). In addition, challenges were acknowledged associated with characterizing O-linked carbohydrates due to a lack of efficient release tools.
When looking at the glycoproteins, it is important to determine glycosylation site occupancy as well as their overall intact protein profiles. One example is Fc pairing in IgG1s. The question is whether an IgG1 is afucosylated on one or two Fc regions. The resulting impact on antibody-dependent cellular cytotoxicity (ADCC) was discussed, and we generally acknowledged a desire to gain further understanding. At present, site-specific glycosylation is routinely determined for MAbs, but the group agreed that it is more difficult to do so with other complex proteins. For example, CE with sodium-dodecyl sulfate gel (CE-SDS) is readily used to determine the degree of occupancy at Asn 297 site for an IgG1. For more complex glycoproteins, currently available methods are not yet optimal for quantitative purposes. Using peptide mapping, you can tell whether a site is occupied, but that is difficult to quantify even using liquid chromatography-mass spectroscopy (LC-MS). In terms of intact protein profiling, the forum recognized that the ability to characterize intact glycoproteins is in its infancy and acknowledged a need for better tools.
THE AFTERNOON SESSION
The afternoon session’s goal was to discuss regulators’ and industry’s current understanding of the structure–function relationship for glycosylated therapeutic proteins and develop a decision matrix to determine the relevance of glycosylation on biologic properties (potency, clearance, immunogenicity) of a given therapeutic.
Presentations: The session covering regulatory perspectives on carbohydrate structure–function relationships was chaired by Wassim Nashabeh (Genentech) and Blair Fraser (FDA CDER):
“Biological Relevance of Glycosylation of MAbs,” by Jun Park (FDA)
“Approach Towards Glycoform Diversity,” by Kurt Brorson (FDA)
“Fc Carbohydrate and Clearance,” by Andrew JS Jones (Genentech, Inc.)
Key Workshop Questions in the afternoon workshop discussion panel included the following:
What are the primary considerations in assessing biological relevance of glycosylation for a given therapeutic? How do we conduct supporting studies?
What key structure–function attributes are well understood and applicable within a given class of protein. These include the role of carbohydrate biological functions (e.g., Fc carbohydrates on antibody effector functions), the role of carbohydrate
on clearance (e.g., sialic acid in therapeutic clearance), and the role of Fc glycans in antibody clearance.
What correlations have been observed between in vitro data and clinical observations?
What are the primary considerations in providing appropriate controls on glycans (e.g., specifications, action limits — no control needed)?
Lot-Release Testing: Characterization methods and routine lot release are generally quite different. For characterization, it is preferable to use a number of orthogonal techniques to fully characterize a carbohydrate/glycoprotein in many different aspects. Lot release is quite different: We look for glycosylation to be similar to our experience. For those reasons, the choice of method for detecting and quantifying carbohydrates depends on the purpose of the testing and intended use of the method.
Two key factors to consider in lotrelease testing are whether a test elucidates or illuminates a key functional attribute or a key manufacturing susceptibility. In addition to characterization and lot-release testing, in-process and stability testing of carbohydrates may also need to be considered. Typical testing strategies for MAbs and other recombinant therapeutic proteins (RTPs) were nicely summarized in Kurt Brorson’s talk (Table 1). It is important to note that product-specific exceptions do exist. Among the many analytical tools, HPLC and CE are the two most widely used tools for routine lot release. As for detection modes, labeling methods are generally more robust than electrochemical detection technologies.
THE CMC STRATEGY FORUM SERIES
The purpose of the CMC Strategy Forum series is to provide a venue for biotechnology/biological product discussion. These meetings focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of such products and thereby foster collaborative technical and regulatory interaction. The forum committee strives to share information with regulatory agencies to assist them in merging good scientific and regulatory practices. Outcomes of the forum meetings are published in this peer-reviewed journal with the hope that they will help assure that biopharmaceutical products manufactured in a regulated environment will continue to be safe and efficacious. The CMC Strategy Forum is organized by CASSS, an International Separation Science Society (formerly the California Separation Science Society), and is cosponsored by the US Food and Drug Administration (FDA).Table 1: Typical testing strategies; MoA= method of action, RTPs = recombinant therapeutic proteins, MAbs = monoclonal antibodies
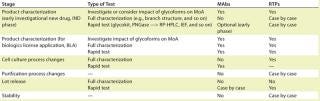
Table 1: Typical testing strategies; MoA= method of action, RTPs = recombinant therapeutic proteins, MAbs = monoclonal antibodies ()
In addition, group consensus said that glycan profiling is the best approach for getting a “fingerprint.” The technique is widely used throughout process development for characterization, comparability, and release testing. Still, it is desirable to be able to look at the end product: a protein with carbohydrates attached to it. Therefore, our goal is to get to a level where we can look at these glycoproteins directly. The group agreed that further development of analytical tools would be desirable in this arena.
DISCLAIMER
The content of this manuscript reflects discussions that occurred during the CMC Forum workshop in addition to the personal viewpoints and experiences of the authors. This document does not represent officially sanctioned FDA policy or opinions and should not be used in lieu of published FDA guidance documents, points-to-consider documents, or direct discussions with the agency.
Structure–Function Relationships: Primary to assessing the biological relevance of glycosylation of a particular therapeutic is the question: “Is it required for clinical efficacy or safety?” Early in biopharmaceutical development, a company simply doesn’t have a database of clinical studies to answer that question. At the beginning, it is difficult even to formulate relevant structure– activity relationship questions. Platform knowledge can be instructive, especially if you are working with an antibody. For example, if it is known that ADCC is important, then maintaining proper glycosylation for Fc receptor binding is also important (1). It is important for the industry to watch closely what is happening in the field of immunogenicity because immunoglobulin sialylation influences proinflammatory and antiinflammatory responses (2).
For therapeutic proteins, the situation is more complex, often with no precedent molecule from which a company can create a guidepost. To help frame “glyco-importance” assessments, the forum developed a decision tree as an aide to making early decisions about glycoform control strategy (Figure 1).
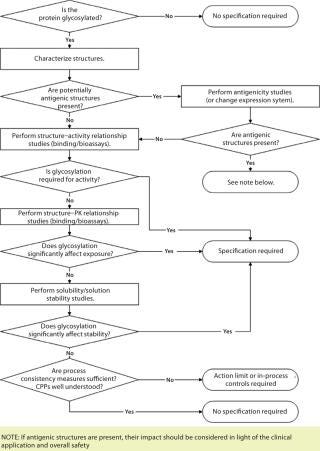
Figure 1: ()
The type of analysis — or even whether a specification would be required — ultimately depends on the breadth and quality of supporting data for any concern addressed in that flow chart. This is where good carbohydrate characterization data and structure– activity relationship studies prove invaluable. However, in vitro testing cannot be the complete arbiter. There is no replacement for actual clinical experience, so the decision tree must be revisited as experience is gained with a biopharmaceutical product. CDER’s Patrick Swann summed it up this way: “You will need the supporting studies to show that your glycosylation is appropriately controlled for the mechanism you have identified by that time.”
Discussion of correlating in vitro test results with clinical observations dealt heavily with immunoglobulin activities such as ADCC and carbohydrate distribution but also addressed biopharmaceutical clearance. The group recognized that challenges remain in establishing such correlations due to noise typically observed in clinical studies. But one identified approach could be a retrospective analysis of in vivo nonclinical or clinical samples as illustrated in Figure 2.
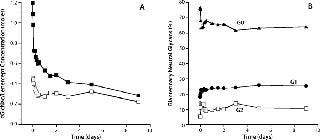
Figure 2: ()
Although linking in vitro activity measures with clinical outcomes has not been well established, clinical data clearly demonstrate a lack of effects on glycoprotein clearance due to Fc glycosylation distribution. Data comparing human and cynomolgus monkey pharmacokinetics for a receptor Fc fusion protein (Lenercept again) highlighted these clearance issues. Correlation between clearance and receptor glycosylation distribution was observed in both species, but Fc glycosylation distribution was unchanged. A precipitous drop in terminal neutral carbohydrates from the whole molecule (expressed as N-acetylglucosamine divided by the product measured in moles) was observed during the initial clearance phase (Figure 2A). It is an effect of clearance throughout interactions of exposed receptor glycosylations with hepatic asialoglycoprotein receptors. Conversely, distribution of Fc glycans (principally neutral glycans) was constant over the study’s duration (Figure 2B) because they are “shielded” from the receptor by the CH2 domain.
The forum generally agreed that variations in normal glycosylation patterns for IgG1s do not affect IgG1 pharmacokinetics, so no limits are needed to control pharmacokinetics (3). Limits might be necessary on Fc glycosylation to ensure consistent effector function, if that is required for an IgG’s mode of action.
Amgen’s Aranesp (darbepoetin alfa) and Genentech’s TNKase (tenecteplase) products were raised as examples of molecules engineered with different glycosylation patterns to increase their biological half-lives. Not only did both molecules demonstrate the in-vitro–in-vivo connection, but they also demonstrated that the effects could be “engineered into” a biopharmaceutical product.
We discussed correlation of in vitro data withw clinical safety indications, paying particular attention to N-glycolyl neuraminic acid (NGNA) and galactose (α1-3) galactose (αGal) structures. Both items have been implicated as potential immunogenic structures. Although good evidence suggests that NGNA is no concern, the story with aGal is not so clear. ATryn human antithrombin III produced by transgenic goats for GTC Biotherapeutics has high levels of NGNA, but it was found to be safe and efficacious in clinical trials and approved for the market. A point was also raised that most nonvegetarian humans have measurable levels of NGNA on their serum glycoproteins obtained through the dietary salvage pathway. It was also noted that a high prevalence of preexisting antibodies target the αGal epitope. Antibodies can increase clearance of a biopharmaceutical, but that effect can be overcome by increased dosing. By contrast with pharmacokinetic or safety observations, very few data were available to correlate in vitro and clinical efficacy readouts. Observation of increased ADCC activity in vitro has not been well correlated with any clinical outcomes. Primary Considerations
Attendees of this CMC Strategy Forum concluded that the primary consideration in providing appropriate controls on glycoprotein pharmaceuticals is the structure–function relationship of their glycosylation. Full characterization of glycodistribution along with its impacts on mode of action, pharmacokinetics, and safety considerations should drive the extent and type of control strategy needed for a given molecule. Selection of appropriate tools for release and stability must be based on appropriate characterization data, whether the goal is to control a specific glycosylation feature critical to structure/function or to control process consistency. Control strategies will evolve as relevant knowledge of both molecule and processes are gained throughout the life-cycle of a therapeutic glycoprotein product — as well as following new developments in the field of glycobiology.
About the Author
Author Details
Corresponding author Joseph Siemiatkoski is director of analytical development for Percivia LLC, 1 Hampshire Street, Cambridge, MA 02139-1578; 1-617-579-7947; www.percivia.com. Corresponding author Stacy Ma is senior director and head of development products and external quality at Genentech (a member of the Roche Group), 1 DNA way, South San Francisco, CA 94080, 1-650-225-1000; www.gene.com. Jun Park is a biologist, Kurt Brorson is a staff scientist, and Patrick Swann is deputy director of FDA/CDER’s Division of Monoclonal Antibodies, Office of Biotechnology Products, Office of Pharmaceutical Science. Lorna D. McLeod is a contributing editor for BioProcess International, [email protected].
1.) Shields, RL. 2002. Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human Fc?RIII and Antibody-Dependent Cellular Toxicity. J. Biol. Chem. 277:26733-26740.
2.) Kaneko, Y, F Nimmerjahn, and J. Ravetch. 2006. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 313:670-673.
3.) Jones, AJS. 2007. Selective Clearance of Glycoforms of a Complex Glycoprotein Pharmaceutical Caused By Terminal N-Acetylglucosamine Is Similar in Humans and Cynomolgus Monkeys. Glycobiol. 17:529-540.
4.) Chung, CH, B Mirakhur, and E. Chan. 2008. A High Prevalence of Hypersensitivity Reactions to Cetuximab Has Been Reported in Some Areas of the United States. NEJM 358:1109-1117.
You May Also Like