Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Nonhuman proteins, heterologous proteins, and even small differences in protein conformation can lead to an immunogenic reaction. That is the case with antiidiotipic antibodies, which react against determinants of an antibody’s antigen-binding region (as depicted here). (HTTPS://ISTOCKPHOTO.COM)
Available literature abounds with case studies describing detection and identification of host cell proteins (HCPs) and other process-related impurities. In the previous installment of our review, we analyzed noteworthy studies, highlighting what they revealed about HCP immunogenicity and calling attention to topics that require further investigation. In this final installment of our four-part study, we focus on HCP risk assessment. We explore current and emerging strategies for immunogenicity prediction, then draw out key insights from the past 40 years of literature on immune responses to HCPs.
Available Analytical Methods
The quantity of HCPs within a drug substance often represents a “good strategic control” for gauging the effectiveness of biopharmaceutical purification processes. However, because many HCPs have detrimental effects on therapeutic proteins, we must increase our understanding of biomanufacturing methods to maintain well-controlled processes that generate products with standard quality attributes. The goal is to minimize threats to patient health by assuring the safety of a biologic for intravenous (IV) administration.
Detection and Identification: Several criticisms can be leveled against enzyme-linked immunosorbent assays (ELISAs) for HCP detection (8). Such assays show reduced detection capability for low-concentration HCPs, and concerns can arise relating to coverage of the HCP spectrum. However, this analytical method detects many immunogenic proteins easily, especially those eliciting high biological responses. In the end, ELISAs based on polyclonal antibodies derived from experimental animals might be the best available option for HCP detection.
Methods such as fluorescent labeling and separation by two-dimensional (2D) electrophoresis with liquid chromatography and tandem mass spectrometry (LC-MS/MS) can measure low HCP concentrations in a given sample. However, a high concentration of an HCP does not necessarily indicate significant potential for immunogenicity, induction of IgE responses, or anaphylaxis. Likewise, a low concentration does not mean that an HCP will be safe for patients, and even low-abundance HCPs can be detrimental for therapeutic proteins through interaction with and exposition of “new” immunogenic epitopes. Thus, MS/MS should be applied to identify and quantify impurities to an acceptable level of accuracy (156). Analyzing those data alongside relevant literature and results from subsequent, more-focused experiments can help to correlate HCP identity with immunogenicity potential.
Immunogenicity Prediction: Cell-based assay methods using toll-like receptors (TLRs) and other such proteins could be useful alternatives to MS/MS for immunogenicity prediction (76, 157). Commercial ELISA kits can be applied to measure interleukins (ILs) 6 and 8 in supernatants from microbial-culture media. Using monoclonal antibodies (MAbs) to block specific TLRs, researchers can determine the origins of impurities present in a sample. Impurities that activate pattern-recognition receptors (PRRs) must be primarily considered because they can elicit strong immune responses, releasing inflammatory cytokines or triggering production of antidrug antibodies (ADAs).
Human leukocyte antigen (HLA)–expressing transgenic mouse models hold much promise for impurity testing (158). Because such organisms are bred to lack murine major histocompatibility complexes (MHCs), they facilitate study of human allergies and autoimmune responses to therapeutic proteins (159). Perhaps transgenic models also could be applied to evaluate HCP immunogenicity.
Scientists increasingly are interested in using software to identify immunogenic proteins. For instance, EpiMatrix algorithms (EpiVax) generate immunogenicity scores for specific proteins (160). Although applications of the technology usually have centered on vaccines, researchers are investigating whether particular HLA domains are prone to inducing immunogenic responses. HLA clusters with high scores (above 10) are associated with T-cell responses (161).
The main disadvantage of in silico testing is the large number of false-positive peptides that cannot elicit T-cell responses in vivo or in vitro. Current computational approaches also cannot account for other factors that inhibit immune response, such as T-cell tolerance in host organisms. Despite such limitations, comparative studies of in silico, ex vivo, and in vivo approaches will help to uncover immunogenic properties of host-cell and therapeutic proteins identified by LC-MS/MS or another analytical method.
HCP Risk Management
As Wang et al. note, risk management is critically important to controlling HCPs in drug substances and products (162). Here, we summarize key considerations. Table 1 lists major factors that are associated with HCP immunogenicity. Table 2 plots such factors in a risk-assessment rubric, and Table 3 shows how that tool might be applied for an example HCP. Below, we reflect on key insights presented throughout our review, then list important risk factors.
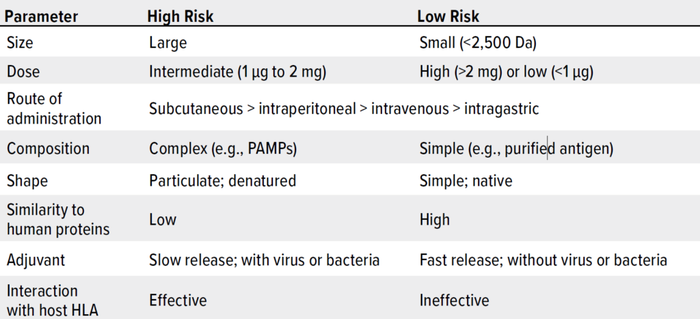
Table 1: Properties of therapeutic proteins that can induce immune reactions (HLA = human leukocyte antigen, PAMPs = pathogen-associated molecular patterns).
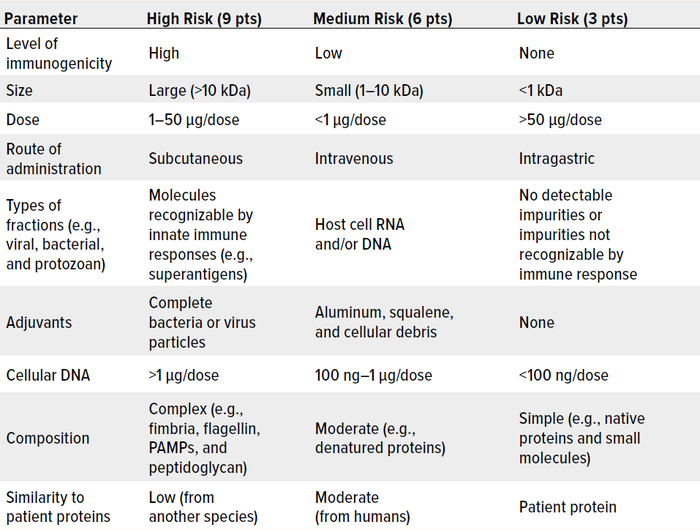
Table 2: A sample risk-assessment framework for host cell proteins (HCPs) and other impurities, with scores of 81 and 27 representing maximal and minimal risk, respectively.
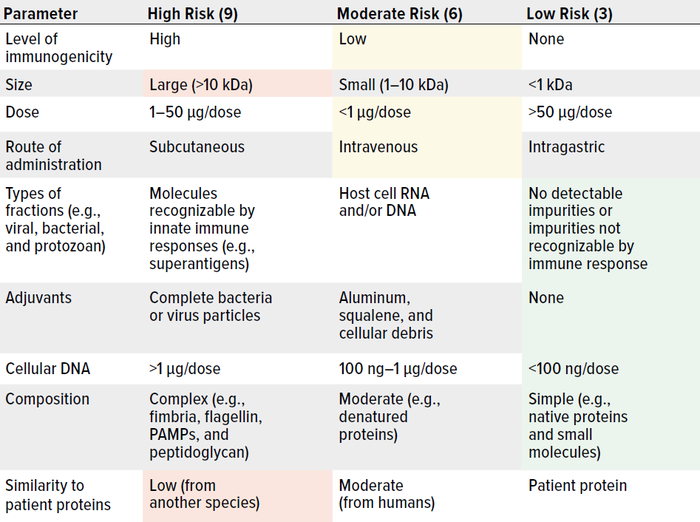
Table 3: Assigning risk to a hypothetical host cell protein (HCP) in a biologic delivered by intravenous injection; scores of 81 and 27 represent maximal and minimal risk, respectively. This example HCP scores 9 + 9 + 6 + 6 + 6 + 3 + 3 + 3 + 3 = 48, which represents moderate risk. (PAMPs = pathogen-associated molecular patterns).
Critical Lessons: Although all enzymatic processes are deleterious to drug substances, such activity can be tracked daily using physicochemical analyses. Knowledge gained from assays must be applied, too. Enzymatically active HCPs must be traced, then inhibited and/or separated from a drug substance. They also can be deleted from the genome in a given expression system.
Bacterial HCPs have proven to be particularly threatening to patients. Although immune responses to yeast and Chinese hamster ovary (CHO) HCPs can compromise therapeutic-protein function, such reactions (to date) have not harmed patients significantly, even when strong IgG and/or IgM responses have been induced.
More than 40 years of clinical evidence demonstrate that protein-drug immunogenicity relates to problems with
• complementarity-determining regions (CDRs, in MAbs)
• nonhuman domains (in MAbs)
• bacterial fractions (e.g., flagellin and pili) that induce production of proinflammatory cytokines
• aggregates
• leachates
• formyl-methionyl residues and other amino acids that activate immune-system detection
• idiosyncratic immune-system behaviors observed in some ethnicities and atopic patients.
Before initiating clinical studies, scientists would do well to track the presence of TLR inductors (impurities) using in vitro IL-induction assays.
HCPs that induce IgE responses can be particularly dangerous for patients. Thus, tracking methods for IgEs, hamster monocyte chemoattractant protein 1 (MCP-1), and similar proteins are of significant interest.
Antibodies often are produced in response to foreign proteins, even in the absence of adjuvants. That said, no evidence exists to suggest cross-reaction with therapeutic proteins.
Although neutralizing antibodies (NAbs) represent a serious problem for biopharmaceutical efficacy, scientists remain unsure about whether HCP neutralization harms patients (by way of proinflammatory cytokines) or merely results in high concentrations of anti-HCP Abs in patient blood.
Biologically active HCPs from nonhuman mammalian cell systems must be detected, identified, quantified, characterized, and separated from drug substances (and/or deleted from the host cell genome) because of similarities in biological features and activities with human cells.
Examples from the literature indicate that certain HCPs coelute with particular therapeutic proteins because of physicochemical properties exhibited during a specific purification process. Not all HCPs coelute with every therapeutic protein. Process and HCP characterization seem to suffice in manufacturing a suitably safe and pure drug substance. Still, HCPs must be reduced to minimal levels in a drug product until we have proof about their potential effects on human health.
Risk Factors: HCP origin is an important consideration during risk assessment. Even small quantities of flagellin (mainly from bacterial cells) can elicit immune responses from T helper type 1 (Th1) cells.
Mammalian-cell proteins can elicit biological responses in humans. Thus, care must be taken to establish tests that are suitable for demonstrating (or discounting) deleterious effects.
Although IgM and IgG responses to HCPs seem not to endanger patients, such responses could influence a product’s pharmacokinetics (PK). That said, we have found no published evidence to verify the possibility.
Pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), alarmins, and other superantigens can induce Th1- and IgE-mediated immune responses, which even in low levels can elicit inflammation and anaphylaxis through release of proinflammatory cytokines, histamine, leukotrienes,
and/or tryptase. Monitoring for such impurities can be important to HCP risk management, enabling testing for immunogenic responses when they are detected.
Route of administration plays a critical role in generating immunogenic and anaphylactic responses to therapeutics with HCPs. Subcutaneous injection usually has resulted in the worst documented cases.
Dose requirements for therapeutic proteins establish how many HCPs and in what concentrations patients might receive upon administration; thus, dosing is critical to immune-response implications. Usually, drug products with relatively small quantities of therapeutic proteins also contain low levels of HCPs. Such levels seem to be safe for patients unless the impurities come from bacterial cells. In the absence of adjuvants and with low concentrations of HCPs, biologic drug products are highly unlikely to elicit exacerbated immune responses.
Dosing frequency plays an important role in immune induction. Whereas vaccines might be administered just a few times over a patient’s lifetime, therapeutic proteins might need to be readministered regularly over a short or long periods. During treatment, chronic diseases can elicit sustained adverse reactions.
Aggregates of therapeutic proteins are implicated in immunogenicity. The same could be true for aggregates that interact with HCPs. Exposition of new immunogenic epitopes might generate complexes that upon immune-system detection could induce production of ADAs. However, this hypothesis remains undemonstrated.
Adverse responses to HCPs observed during clinical trials have occurred when the impurities have exhibited similar physicochemical properties to those of the target proteins. For instance, immunogenicity concerns with abatacept, recombinant human growth hormone (rhGH), and therapeutic MAbs have been associated with macrophage inhibitory cytokine 1 (MIC-1), flagellin, and complexes of mucin protein 1 (MUC-1) with transforming growth factor beta (TGF-β), respectively. Thus, HCP specifications can be set a priori while scientific information increases about a product.
To detect potential for exacerbated immune responses (to therapeutic-protein aggregates, trace host cell impurities, leachables, and the product itself), tolerance tests must be administered to all patients before they undergo treatment. Such testing should occur even when biotherapeutics have demonstrated safety because some patient populations might have idiosyncratic responses.
Improving the accuracy of anaphylaxis-prediction tools can help to diminish or eliminate adverse effects.The biopharmaceutical industry needs to create a verifiable database with official immunological data related to host-cell impurities.
The State of HCP Analytics
Available literature contains plenty of information regarding HCPs and their risks for patient health. Experience also tells us that proteins from infectious cells such as bacteria can exert strong proinflammatory responses, whereas impurities from CHO and other cells that resemble those of our species can interfere with human biological functions. However, the field still lacks a comprehensive map of the main issues surrounding HCPs, topics needing further investigation, and control strategies. The purpose of our review has been to make sense of many years of studies on how HCPs influence biopharmaceutical efficacy and safety. We analyzed case studies that have been critical to the field’s understanding of such impurities, also highlighting information that remains unclear.
Although routine quality control (QC) analyses and stability studies easily can detect molecular-level problems in biopharmaceuticals, effects from highly biologically active HCPs and immunological issues can remain hidden from daily physicochemical analyses until clinical trials are performed. Thus, drug makers must perform orthogonal biological assays (e.g., testing based on monocyte-derived cell lines to measure histamine and cytokine levels), immunological assays, and/or experiments using transgenic mice with HLA allotypes to uncover unexpected adverse reactions before clinical studies begin. Pharmacovigilance during short- and long-term postmarketing studies also should play a critical role in tracking secondary effects, risks, and benefits not observed during trials.
Regarding adjuvant activity, although lipopolysaccharides (LPSs) and CpG sites are not proteins, they still must be controlled and quantified routinely. In animal models, such impurities have induced production of Abs against coadministered proteins. Those findings suggest that other molecules could elicit similar responses in treated patients.
Our review has shown that some issues related to HCPs stem from misunderstandings, insufficient data, or unknown information. Emerging data will be critical in guiding drug makers’ decision-making, minimizing threats to patients, and increasing industry credibility. During purification processes, attention also must be paid to removing highly biologically active molecules that could induce adverse reactions in large and diverse patient populations. We hope that our review triggers further, more-focused studies into whether interactions between therapeutic and host-cell proteins induce immunomodulatory responses. We also hope that researchers will investigate whether studying HCPs truly helps to understand process control.
Acknowledgments
This work was supported by Mexico’s Consejo Nacional de Ciencia y Tecnología (CONACyT), Grant FINNOVA-CONACyT 174104. Authors VPMM and NOP are Sistema Nacional de Investigadores (SNI)-L1 CONACyT Fellows.
Disclosures
Probiomed S.A. de C.V. develops, manufactures, and markets biosimilar products. All three authors are involved in the development of biosimilar products for Probiomed.
References
See part 1 for references 1–34, part 2 for references 35–129, and part 3 for references 130–155.
8 Zhu-Shimoni J, et al. Host Cell Protein Testing By ELISAs and the Use of Orthogonal Methods. Biotechnol. Bioeng. 111, 2014: 2367–2379; https://doi.org/10.1002/bit.25327.
76 Huang LY, et al. Use of Toll-Like Receptor Assays To Detect and Identify Microbial Contaminants in Biological Products. J. Clin. Microbiol. 47(11) 2009: 3427–3434; https://doi.org/10.1128/JCM.00373-09.
156 Bomans K, et al. Identification and Monitoring of Host Cell Proteins By Mass Spectrometry Combined with High Performance Immunochemistry Testing. PLoS One 8(11) 2013: e81639; https://doi.org/10.1371/journal.pone.0081639.
157 Haile LA, et al. Cell Based Assay Identifies TLR2 and TLR4 Stimulating Impurities in Interferon Beta. Sci. Rep. 7(1) 2017: 10490; https://doi.org/10.1038/s41598-017-09981-w.
158 Das P, Abraham R, David C. HLA Transgenic Mice as Models of Human Autoimmune Diseases. Rev. Immunogenet. 2(1) 2000: 105–114.
159 Madsen L, et al. Mice Lacking All Conventional MHC Class II Genes. Proc. Nat. Acad. Sci. (USA) 96(18) 1999: 10338–10343; https://doi.org/10.1073/pnas.96.18.10338.
160 De Groot AS, Moise L. Prediction of Immunogenicity for Therapeutic Proteins: State of the Art. Curr. Opin. Drug Discov. Dev. 10(3) 2007: 332–340.
161 Koren E, et al. Clinical Validation of the ‘‘In Silico’’ Prediction of Immunogenicity of a Human Recombinant Therapeutic Protein. Clin. Immunol. 124, 2007: 26–32.
162 Wang F, et al. Host-Cell Protein Risk Management and Control During Bioprocess Development: A Consolidated Biotech Industry Review, Part 1. BioProcess Int. 16(6) 2018: 42–47, 64; https://bioprocessintl.com/business/risk-management/host-cell-protein-risk-management-and-control-during-bioprocess-development-a-consolidated-biotech-industry-review-part-1.
Víctor Pérez Medina Martínez and Carlos Eduardo Espinosa-de la Garza work in the research and development unit, and corresponding author Néstor O. Pérez works in operations management at Probiomed S.A. de C.V., Cruce de Carreteras Acatzingo-Zumpahuacán s/n, Tenancingo, Estado de México, México. C.P. 52400; 52-55-1166-2305; [email protected]; https://www.probiomed.com.mx.
You May Also Like