Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 15, 2014
Aeras is a nonprofit biotech focused on the development of TB vaccines. Together with our global research and development partners, we have played an integral role in developing approximately half of all TB vaccine candidates in clinical development around the world.
TB is one of the most deadly infectious diseases in the world. It spreads through the air like the common cold. A TB patient with active disease can infect up to 15 people simply by coughing, sneezing, or talking. The emergence and spread of drug-resistant forms of TB are complicating multinational efforts to control the global epidemic.
Because TB is more complex and more difficult than ever to treat, it presents a worldwide health threat. So attacking this challenge requires developing expertise and experience in multiple biologics technology platforms. Aeras approach includes building integrated, state-of-the-art, end-to-end development and manufacturing capabilities for translational development support all under one roof.
Aeras is leveraging these capabilities to assist others in the development of vaccines or other biologics against other diseases. Aeras always focuses from the start on robust processes and high-quality products optimized for late-stage development and commercialization, providing end-to-end support from process and analytical development through manufacturing and fillfinish of both preclinical and Phase 12 clinical trial materials.
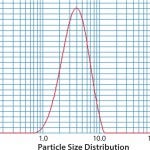
FIGURE 1: Unique unit of operations: spray drying example; particle-size distribution (Jin TH, et al. Vaccine 28, 210: 43694375)
FIGURE 2: Unique unit of operations: stability of spray drying (Jin TH, et al. Vaccine 28, 210: 43694375)
Our team is focused throughout the product development process on ensuring that processes are scalable to meet global manufacturability requirements. Aeras delivers processes that are optimized for large-scale and can support tech transfer for phase 3 and commercial-production volumes and cost of goods targets.
Our experiences with TB vaccine development have required us to develop capabilities with multiple technology platforms, including subunit antigen and viral vaccines, plus recombinant proteins and monoclonal antibodies. We have developed unique capabilities with spray-drying (Figures 1, 2), both in the PD and current good manufacturing practice (CGMP)/aseptic environments, providing development avenues for thermostable product storage, and aerosolized delivery.
End-to-end operations include
extensive process and analytical development capabilities
GMP-compliant manufacturing capabilities (meeting FDA and EMA standards)
BSL2 facility
expertise with live agents and proteins
extensive range of manufacturing platforms
cross-trained, flexible workforce.
Based on our extensive experience with our own vaccine development programs, our team understands your product development challenges. We are ready to collaborate for your success.
Peter Alexander, PhD, is senior director, technical operations at Aeras, 1405 Research Blvd., Rockville, MD 20850; 1-240-599-3085;
[email protected].
You May Also Like