Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

Protein A chromatography has become a widely used platform in monoclonal antibody (MAb) purification. We describe the impact of higher MAb loadings on aggregation and ligand leaching during protein A chromatography, as well as the benefits of high-capacity Protein A resins for high-titer feedstocks. Today, high MAb titers achieved through advanced upstream processing have become a challenge for downstream processing, especially with regards to the expensive protein A capturing step. TOYOPEARL AF-rProtein A HC-650F provides superior MAb capacity and beneficial MAb uptake behavior and can thereby increase capturing productivity.
MAb Adsorption: Because dynamic capacity depends on flow and feed concentration, it was tested at various residence times (RT) and MAb titers. Figure 1 shows the breakthrough curve for TOYOPEARL AF-rProtein A HC-650F at a feed concentration of 10 g mAb/L. The resin shows complete MAb adsorption until breakthrough occurs even for short RT of 1 min. The capacities of more than 100 mg/mL exceed the DBCs of all other base-stable protein A resins.
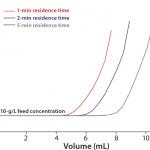
FIGURE 1: Dynamic binding capacity; column: TOYOPEARL AF-rProtein A HC-650F 6.6 mm ID 2 cm L; residence time 1 min, 2 min, 5 min; sample = 10 g/L mAb A; UVmax >2000 mAU
MAb Elution: To evaluate elution properties, the MAb was diluted to a final concentration of 4.75 g/L and spiked with host-cell proteins. Protein A chromatography was conducted in 200-L RoboColumns at a RT of two minutes. The total loaded mass was varied from 10 to 50 mg/mL resin, and columns were washed with 20 CV of loading buffer before elution. Recovery was >95% at pH 3.25. SEC analyses of two elution pools (10 and 50 mg/mL load) revealed similar aggregate contents (0.6%) when referring to the corresponding total protein amount, proving that large amounts of MAb absorbed by high-capacity resins do not in principle enhance the risk for MAb aggregation during elution.
Ligand Leaching: The dependency between the absolute load and protein A leaching at various MAb titers for high-capacity resins was analyzed for 2.5- and 7-g/L concentrated feed streams. The contour plots reveal that the absolute load has little influence on numeric protein A leaching (Figure 2).

FIGURE 2: Contour plots for two load concentrations; protein A leaching is plotted against pH and absolute load (a) 2.5 g/L; (b) 7 g/L.
Considering the obtained results for protein A leaching, aggregate content, and protein adsorption, high titers seem favorable for protein A chromatography. This MAb seemed unaffected with regards to aggregation and was efficiently adsorbed, which reduces protein A cycle time. Ligand leaching was even slightly lower when applying higher titers. The high capacities at high feed concentrations offered by TOYOPEARL AF-rProtein A HC-650F enable fast and efficient capturing.
Regina Römling is a marketing manager, and Judith Vajda, is senior lab specialist at Tosoh Bioscience GmbH; 49-711-13257-0;
[email protected]. Angelika Wacker
is a master student at University of Applied Sciences Mannheim.
You May Also Like