Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Cytiva
 Fast and accurate determination of vaccine titer during manufacturing is important for understanding the performance of a development process and for scaling each process step. Although single radial immunodiffusion (SRID) has been the most commonly used technique for vaccine titer determination, it can be time-consuming and imprecise, requiring up to three days for results. The Pall ForteBio Octet® system offers a simple and direct method for measuring vaccine–antigen–antibody binding, capable of delivering high-precision analysis in as little as three hours.
Fast and accurate determination of vaccine titer during manufacturing is important for understanding the performance of a development process and for scaling each process step. Although single radial immunodiffusion (SRID) has been the most commonly used technique for vaccine titer determination, it can be time-consuming and imprecise, requiring up to three days for results. The Pall ForteBio Octet® system offers a simple and direct method for measuring vaccine–antigen–antibody binding, capable of delivering high-precision analysis in as little as three hours.
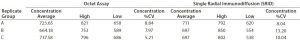
Table 1: Comparison of Octet system with single radial immunodiffusion (SRID); B/Massachusetts influenza virus
Methods
Using the influenza virus as a model system, the relative standard deviation and dynamic range of a vaccine titer assay were measured with the Octet system and compared with results from using SRID. Polyclonal serum antibodies were loaded onto Octet biosensors, and their binding measured to epitopes presented by an inactivated virus standard to construct a standard curve. A linear range was established at 5–75 µg/mL. Vaccine samples were then measured on the Octet system and their concentration interpolated from the standard curve. Finally, vaccine titers obtained in parallel by SRID were compared with those from using the Octet system (Table 1).

Figure 1: Work flow for vaccine potency assay
Results
The vaccine titer delivered by the Octet system offers higher quality data and greater reproducibility over a wider dynamic range than delivered by the SRID method. In addition, the Octet system sped the data acquisition time dramatically. By analyzing the vaccine sample directly in 96- or 384-well microplates, researchers observed that the Octet system enabled a high-throughput analytical method with less required sample preparation. The hemagglutinin (HA) content measured by the SRID method needed to first be lysed with Zwittergent 314 detergent from MilliporeSigma. By contrast,
vaccine samples needed only to be diluted to run on the Octet system, thereby leaving them in their native state without the use of detergents. This further expands the molecule types that can be analyzed on the Octet system to whole virus, split virions, and recombinant HA vaccine samples.
Adding to the flexibility of the Octet system in the vaccine development process, the binding assays also could be used as stability indicators. The sensitivity of the Octet system could be used to distinguish between native and inactivated antigens under accelerated degradation conditions such as high temperature, extreme pH, and oxidative conditions. As antigens undergo conformational or other changes in response to those stresses, the corresponding loss in binding activity can be characterized by the Octet system.
David Wheatley is principal scientist, and Debi Saunders is a staff scientist at Pall Life Sciences. David Apiyo is a marketing applications manager at Pall ForteBio; [email protected].
You May Also Like