Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
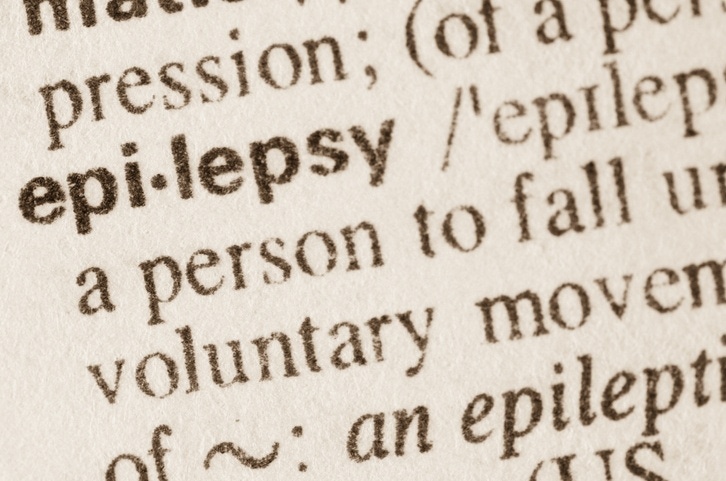
Cobra Biologics will produce the AAV viral vector for Combigene’s epilepsy gene therapy from its facilities in Keele, UK.
Nordic gene therapy company Combigene’s CG01 candidate is based on an adeno-associated viral vector (AAV) which administers a combination of neuropeptide Y (NPY) and its receptor Y2 directly to the part of the brain where epileptic seizures begin.
The project has received funding from the European Union’s Horizon 2020 research and innovation program and the manufacturing process was developed at the UK’s Cell and Gene Therapy (CGT) Catapult.
Image: iStock/aga7ta
But now Swedish contract development and manufacturing organization (CDMO) Cobra Biologics has entered into a Master Service Agreement with Combigene to make clinical materials of the epilepsy gene therapy. If the candidate reaches approval, Cobra will be contracted to make commercial quantities of the product.
“Cobra is one of only a handful of CDMOs globally who offer both viral vector manufacture and plasmid DNA production, making us perfectly placed to support this project,” a Cobra spokesperson told this publication.
“The work for will be carried out at our Keele site in the UK,” the spokesperson said, adding “the project will take advantage of our ongoing £30 million [$38 million] Advanced Therapy expansion which has resulted in more space, equipment and a considerable recruitment drive.”
Cobra began an expansion of its manufacturing facilities in 2017 to meet increased demand of gene and immunotherapy companies. The projects included adding viral vector Phase III and commercial manufacturing capabilities at the Keele plant – located between Birmingham and Manchester – and doubling capacity for DNA plasmid production at the site in Matfors, Sweden.
Karin Agerman, chief R&D officer at CombiGene, said the choice of Cobra “marks a significant milestone in the CG01 project and an important step towards clinical studies. Cobra meets all of our selection criteria and has shown itself to be a company that responds quickly and is easy to collaborate with.”
The firm has also selected Michigan, US-based Northern Biomedical Research (NBR) as the contract research organization (CRO) partner for CG01, and a small pilot study is set to begin.
“This is necessary to give material to our collaborators that will develop the bioanalytical tools to be able to evaluate the effect of CG01,” CombiGene says. “The full-scale biodistribution and toxicity studies will be carried out with material produced by Cobra according to the process which will subsequently be used for GMP manufacturing.”
You May Also Like