Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
May 28, 2020
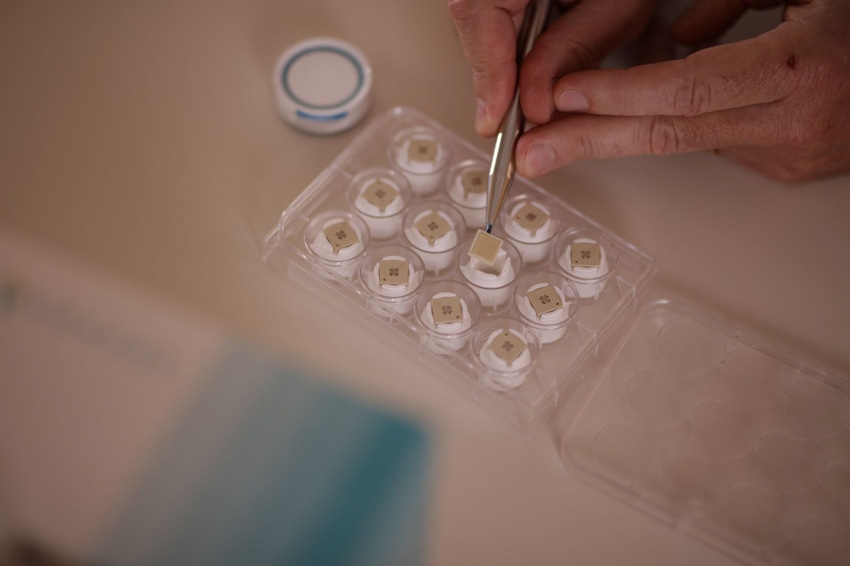
Merck & Co. will use Vaxxas’ delivery patch technology in one of its vaccine candidate development programs.
Brisbane, Australia based Vaxxas announced the agreement today, explaining the US drug firm exercised an option under a co-development accord signed in 2012.
Vaxxas CEO David Hoey told us the technology – known as the High Density Microarray Patch (HD-MAP) platform – “uses an ultra-high density array of very short projections applied briefly to the skin to rapidly deliver vaccine to the abundant immune cells immediately below the skin surface.”
The high-density of the array provides high surface area for vaccine coating and is designed to further activate immune response upon application.
He added, “This technology can be used to deliver any vaccine, and is the only patch technology that has been clinically validated (using influenza vaccine) to be capable of inoculating up to six times more people using the same amount of vaccine.
“Vaccines are printed onto the projections and, in this dry format, can be stable outside cold-chain.”
Details of Merck’s vaccine are not being made public. Hoey told us “Merck has requested that we not disclose the specific vaccine we are commercializing with them.”
According to a Nature Biotechnology report, the HD-MAP platform has been used to deliver Merck’s human papillomavirus (HPV) vaccine Gardasil into mouse-ear epidermal and dermal skin in preclinical studies.
Sterile manufacture of clinical materials currently takes place in cleanroom at Vaxxas’ facility in Queensland, Australia, under a validated aseptic process. At present the facility can produce around 1,000 products per day suitable for early stage clinical use.
In a separate announcement, Vaxxas revealed that German contract development and manufacturing organization (CDMO) Harro Hofliger will develop the world’s first high-throughput aseptic line for vaccine-HD-MAP production.
The firm said the collaboration “lays the engineering groundwork for capital expenditure investments of up to $25 million over the next three years.”
Hoey told us “The pilot line being engineered by Harro Hofliger will be used to supply later-stage clinical studies. At 1/5th scale, the initial pilot-line will have capacity of around 200,000 units/day, which corresponds to over 50 million units per year depending on operating scheduling.”
You May Also Like