Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
November 2, 2020
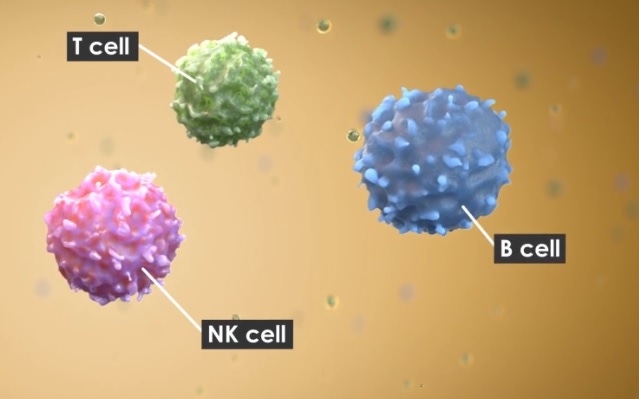
Once the deal goes through, the French pharma giant will add an allogeneic natural killer (NK) cell therapy platform to its immune-oncology capabilities.
In cell therapy development, industry has seen some success with T-cells, most notably in the approvals of the chimeric antigen receptor T-cell therapies Kymriah and Yescarta. T cells work by spurring patients’ adaptive immune system, which learns to remember threats and eliminates them when they return.
However, NK cells, which are part of our innate immune system, are increasingly being looked at to defend against foreign invaders without such prior instruction. Furthermore, they can be modified into CAR-NK therapies and used to target solid tumors and have some advantages over CAR-T therapies mostly in terms of safety.
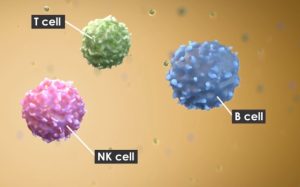
As such, Sanofi has made a bid to buy allogeneic NK-cell based therapy developer Kiadis for €308 million ($358 million). The firm is developing immunotherapies against multiple solid tumor and hematological malignancies through isolation and culture of PBMCs using a nano-particle technology.
The nanoparticles are produced from cells engineered with specific membrane bound proteins, which stimulate NK cells to rapidly expand by generating large numbers of cytotoxic NK cells. The tech is being evaluated as a standalone therapy and in combination with others including stem cell transplant and monoclonal antibodies.
“We believe the Kiadis ‘off the shelf’ K-NK cell technology platform will have broad application against liquid and solid tumors, and create synergies with Sanofi’s emerging immuno-oncology pipeline, providing opportunities for us to pursue potential best-in-disease approaches,” said John Reed, global head of R&D at Sanofi.
The move comes months after Sanofi inked a deal with Kiadis to license the pre-clinical K-NK004 program for potential combination for multiple myeloma.
You May Also Like