Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
CDMO AGC Bio is among the 17 companies selected by Japan’s Ministry of Economy, Trade, and Industry (METI) to support pandemic-ready vaccine manufacturing.
As part of Japan’s fiscal 2021 budget, the ‘Biopharmaceutical Manufacturing Base Development Project for Strengthening Vaccine Production System’ invited applications from academia and industry to help secure bioproduction for future pandemics.
The aim is to develop a Japan-based production network – from formulation, to fill-finish – with dual-use equipment that manufactures biopharmaceuticals that meet corporate needs during normal times, and switches to vaccine manufacturing in the event of an infectious disease pandemic.

Image: DepositPhotos/
TriDraw
The Japanese government received 41 applications during March and May this year and after a “rigorous examination by a third-party committee of external experts,” 17 proposals have been accepted, receiving a total of ¥ 226.5 billion ($1.56 billion).
The full 17 companies can be found here (in Japanese), but some examples of recipients include mRNA and viral vector maker Takara Bio, ingredient and technology firm Nipro Pharma, single-use bag and tube assembly Fujimori Industry, the National University Corporation Hiroshima University, and big biopharma Daiichi Sankyo.
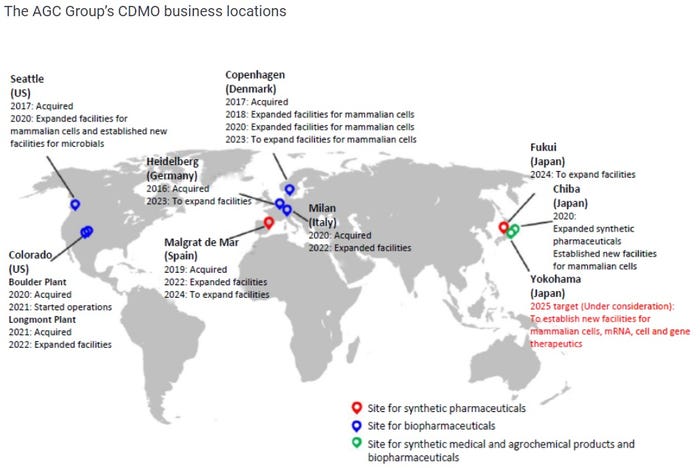
Glass and chemical manufacturer AGC was also selected by METI. The firm, through acquisitions including CMC Biologics and Biomeva, operates a biologics contract development and manufacturing organization (CDMO) with significant operations in Europe and the US. This tender win will accelerate an expansion of its biopharmaceutical CDMO business in Yokohama, Japan.
“The additional capabilities AGC is considering for the AGC Yokohama Technical Center consists of CDMO services for mRNA pharmaceuticals, cell and gene therapeutics, and protein-based biopharmaceuticals made using mammalian cell cultures, which also can be applied for the manufacture of vaccines in the event of a pandemic,” the firm said in a press release.
“With the Bio-CDMO know-how that the Group has cultivated at its multiple sites, Japan, the US, and Europe, the Group aims to early expand its services in Japan, thereby adding to the domestic biopharmaceutical development and manufacturing capacities, and a more robust production network.”
AGC’s full manufacturing network can be seen below:
You May Also Like