Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
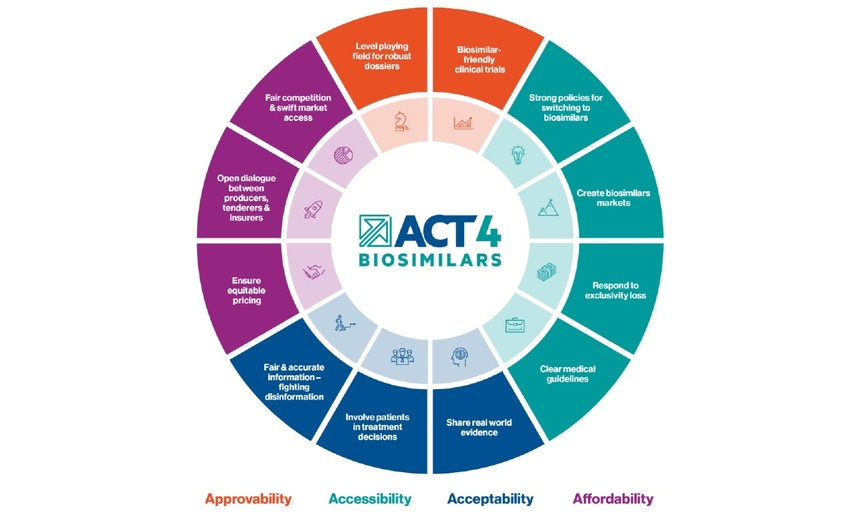
The 12 goals within Sandoz’ Act4Biosimilars initiative hope to increase the global adoption of biosimilars by at least 30% in over 30 countries by 2030.
Novartis’s Sandoz division is a pioneer in the biosimilars space, being the first company to receive approval of a biosimilar in the US through Zarxio, a version of Amgen’s Neupogen(filgrastim), in 2015. It has also been an advocate of these medicines, which have the potential to reduce the cost of medicine for patients and payors.
Now the firm has launched Act4Biosimilars, a roadmap hoping to increase patient access to biosimilars globally with a mission to increase global adoption of biosimilar medicines by at least 30% in 30+ countries by 2030.

“Act4Biosimilars is the first biosimilar initiative with a global scope, bringing together international stakeholders united in a common mission to bring the benefits and value of biosimilars to more patients in more countries, a Sandoz spokesperson told BioProcess Insider.
“By co-creating the Action Plan with experts from patient organizations, trade associations, think tanks, government bodies and professional organizations, Act4Biosimilars will be creating and implementing an Action Plan, based on 12 pre-defined goals, that provides strategies, tools and guidance to support improving the approvability, accessibility, acceptability and affordability of biosimilars across the 30 countries and beyond.”
The 12 goals focus on approvability (1. A level playing field for robust dossiers, 2. Biosimilar-friendly trials), accessibility (3. Strong policies for switching, 4. Create biosimilars markets, 5. Respond to exclusivity lost, 6. Clear medical guidelines), acceptability (7. Share real world evidence, 8. Involve patients in treatment decisions, 9. Fair and accurate information – fighting disinformation), and affordability (10. Ensure equitable pricing, 11. Open dialogue between producers, tenderers, and insurers, 12. Fair competition and swift market access).
According to the spokesperson, 30% constitutes the minimum combined worldwide growth in biosimilar adoption that Act4Biosimilars aims to achieve between now and 2030. “This objective embraces and takes into consideration the varied and uneven biosimilar adoption status across the 30 target countries, as well as the diverse characteristics of each health system and the dynamics of each market.”
The spokesperson did not respond in terms of how this would relate to cost savings, but told us the data measuring success will be “visualized on Country Indicator Maps housed on the Act4Biosimilars website. This gives the user an opportunity to explore how each of the 30 countries is progressing with our mission of increasing global adoption of biosimilars by at least 30% in 30+ countries by 2030.”
To further the campaign, Sandoz is looking for organizations and individuals who share an appreciation of biosimilars’ ongoing contribution to healthcare systems and a drive to further spread the promise of biosimilars.
With biosimilars being available in the US for seven years and in other regions for much longer, we asked if the need for this campaign shows the promise of biosimilars has failed.
“Act4Biosimilars’ 30 target countries are at varying stages of biosimilar adoption, ranging from those aspiring to introduce biosimilars for the first time to those where biosimilar adoption remains active and ongoing,” the spokesperson said.
“Act4Biosimilars aims to champion the relevant strategies and the necessary tools for every country, taking into consideration insights and lessons learned from existing markets as well as the characteristics of the target countries’ local healthcare system and status of biosimilar adoption. We believe that advocacy initiatives for biosimilars are needed in all parts of the world, even in Europe which is widely viewed as the most successful biosimilar market worldwide.”
You May Also Like