Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
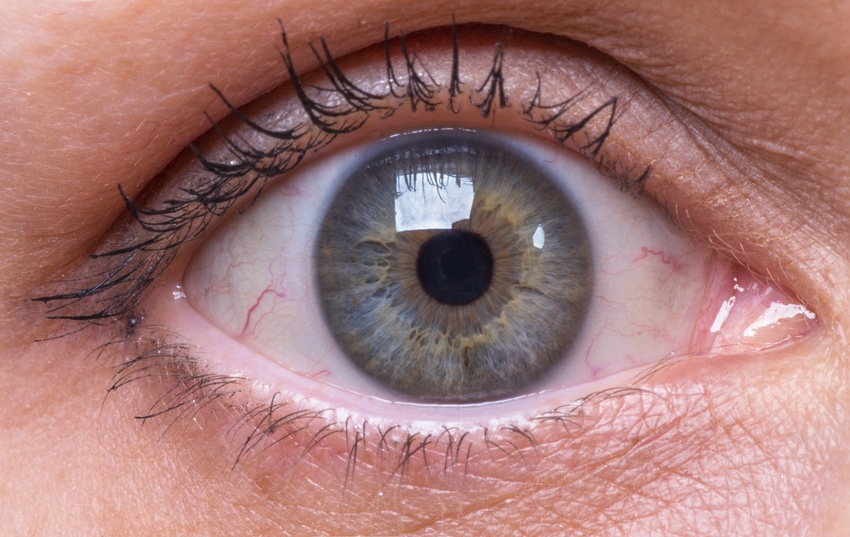
Novartis will add a gene therapy candidate in Phase II trials for an advanced form of dry age-related macular degeneration through the addition of Gyroscope Therapeutics.
Novartis will gain GT005, an Adeno-Associated Virus-2 (AAV)-based gene therapy candidate intended to be a one-time treatment for geographic atrophy (GA) – an advanced form of dry age-related macular degeneration (AMD) that leads to progressive and irreversible vision loss – through the proposed acquisition.
Novartis is one of the leaders in the gene therapy space, having bought gene therapy pioneer AveXis in May 2018 for $8.7 billion, and later incorporating the business under its Novartis Gene Therapies division in September 2020.
[icegram messages="59886"]
Having already seen success through the commercialization of Spark Therapeutics’ Luxturna (voretigene neparvovec) outside of the US, the deal is the latest in a string of acquisitions in the ophthalmology gene therapy space.
In September 2021, the firm acquired Arctos Medical for an undisclosed fee, gaining a preclinical optogenetic AAV gene therapy program and technology platform. And in October 2020, the firm added two preclinical programs and delivery technology for treating inherited retinal dystrophies and geographic atrophy through the addition of Vedere Bio.
“With our own pioneering research in ocular gene therapies and our experience gained from bringing Luxturna to inherited retinal dystrophy patients outside of the US, Novartis has a well-established expertise in ocular gene therapies that will position us well to continue developing this promising one-time treatment,” said Marie-France Tschudin, president of Novartis Pharmaceuticals.
“This acquisition is one more step forward in our commitment to delivering innovation in ophthalmology to treat and prevent blindness worldwide.”
Gyroscope’s parent company Syncona Limited will receive $800 million upfront from the Swiss Pharma giant but could receive a further $700 million upon the achievement of certain customary milestones related to clinical development, regulatory approvals and reimbursement.
“In five and a half years, enabled by collaborations with four leading UK universities, we have taken Gyroscope from a concept to a potential treatment for geographic atrophy secondary to AMD, a leading cause of blindness with no approved therapies. Gyroscope is now an international company with world-class management, positive clinical data, proprietary surgical and manufacturing platforms, and a team of nearly 200 people,” said Chris Hollowood, chief investment officer of Syncona Investment Management Limited.
“The structure of the transaction will provide us with ongoing exposure to Gyroscope’s development and the potential for significant additional returns, subject to certain milestones. We look forward to seeing Gyroscope fulfil its potential during the next phase of its growth with Novartis, who has an extensive track record in gene therapy and ophthalmology and are ideally placed to complete the journey of taking this transformational therapy to patients.”
You May Also Like