Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
April 8, 2020
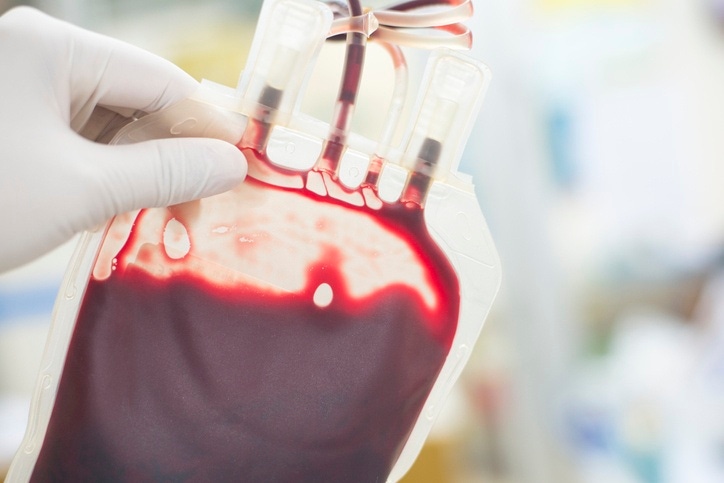
Takeda says COVID-19 therapy accord will work on TAK-888 and other plasma-derived meds developed by its partners.
The Japanese drug firm teamed up with CSL Behring, Biotest, BPL Group, LFB and Octapharma this week. The aim is to turn blood plasma from recovered patients into treatments for COVID-19.
The initial focus will be TAK-888, an anti-SARS-CoV-2 polyclonal hyperimmune immunoglobulin medicine. But a Takeda spokeswoman told us the idea is to work on all in-progress development efforts.
Image: iStock/toeytoey2530
“Companies collaborating as part of this alliance are now bringing together their respective investigational programs focused on COVID-19, with the aim of developing a single unbranded potential therapy.
“This includes the work done in the TAK888 program as well as other investigational programs other participating companies had underway,” she added.
The plan is to leverage members’ plasma collection capabilities as well as those of external sources, Takeda says.
“The companies participating in the agreement have extensive plasma collection networks throughout the world as part of our vital roles in developing plasma-derived therapies for rare and serious conditions.
“We are also working with regulatory bodies, healthcare systems and institutions around the world to access plasma through other means, including an effort to find the most effective, efficient and safest way to locate recovering COVID-19 patients who may be willing to donate plasma,” the spokeswoman said.
Takeda shared details of TAK-888 last month. The firm will make the product – a concentrate of antibodies collected from recovered patients – at its facility in Georgia, US.
Collaboration members will share production of other COVID-19 therapies developed.
Takeda’s spokesperson said, “The alliance plans to use plants and processes already licensed for the isolation of the targeted antibodies and the required manufacturing steps. Where exactly this will occur has not yet been decided, but we will provide more detail at a later time.”
Takeda expects products developed under the collaboration to enter clinical trials before the end of the year. But, exact timelines and rate of progress will depend on the ability to source materials the spokeswoman said.
“We are moving quickly and expect to enter the clinic in the coming months, with the goal of making the product available to COVID-19 patients at high risk as soon as possible. Our biggest unknown factors are rate of convalescent plasma collection and regulatory approval timeline.”
You May Also Like