Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
CRISPR Therapeutics says pharma must realize that an allogeneic approach is the only sustainable way of “doing cell therapies across broad populations.”
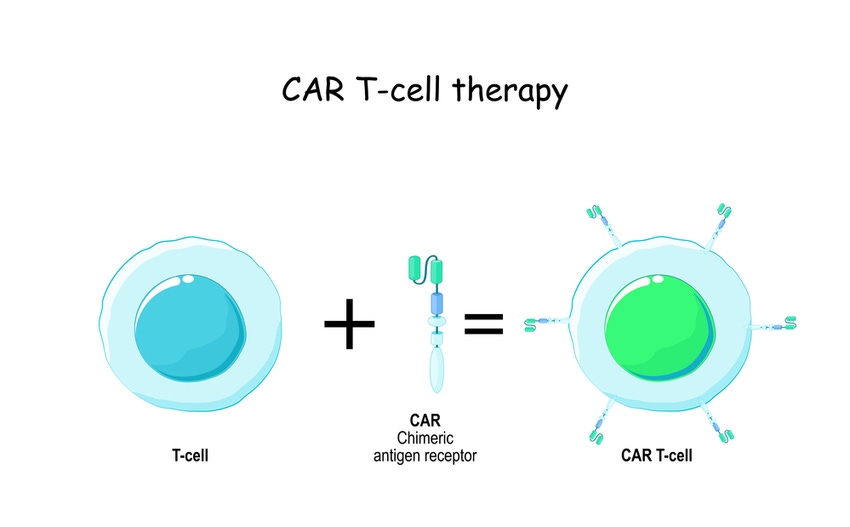
While the market has seen a flurry of chimeric antigen receptor (CAR) T-cell therapy approvals – Novartis’ Kymriah (tisagenlecleucel), Gilead’s Yescarta (axicabtagene ciloleucel) and Tecartus (brexucabtagene autoleucel), Bristol Myer Squibb’s Abecma (idecabtagene vicleucel) and Breyanzi (lisocabtagene maraleucel), J&J’s Carvykti (ciltacabtagene autoleucel) – these have all been autologous products, made by taking, reengineering, and reintroducing a patient’s own cells.
However, a number of developers are taking an allogeneic, or off-the-shelf, approach to cell products. These use master cell banks (MCBs) based on a donor’s cells to make transplanted therapies. As these are not personalized, one advantage over autologous is the relative ease to mass-produce such products.
Image: DepositPhotos/edesignua
One such biotech is Switzerland’s CRISPR Therapeutics, which focuses on a range of allogeneic CRISPR/Cas9 gene-edited CAR-T cell therapies, including β-thalassemia and sickle cell disease programs being developed with Vertex Pharmaceuticals and a series of oncology programs.
Speaking earlier this month at the Morgan Stanley Annual Global Healthcare Conference, CRISPR’s CEO Samarth Kulkarni lauded the potential of allogeneic, especially against cancer.
“The fight against cancer has gone from a cocktail of small molecules all the way to target antibodies. But we’ve seen the limitations of antibodies and ADCs,” he told stakeholders, adding the “world cell therapies” is the next stage.
However, “the only sustainable way of doing cell therapies across broad populations is going to be allogeneic cell therapies, unless we crack the code on bedside autologous therapies. And within allogeneic therapies, now there’s a lot of data around T cells, NK cells, […] alpha, beta or gamma, delta, etc.”
He claimed CRISPR has shown allogeneic CAR-Ts can have impact in solid tumors – “we had the first instance of a complete response in a solid tumor with an allogeneic CAR-T which people said would require four noble prizes before we get there – and hopes to soon show durable remissions
“We’re making dramatic progress in cell therapies. What we’re going through from an investor standpoint right now is a bit of the initial excitement hype, the phase of disillusionment. But once pharma wakes up to it and pharma is coming around to allergenic cell therapies, you’re going to see another sort of cycle where there’s going to be a tremendous investment in cell therapies.”
He added his firm is “at the vanguard” of this space, before praising what he believes is “the most advanced manufacturing setup that you’d ever see for allogeneic cell therapies in Framingham, in Massachusetts which is operational now.”
The facility was commissioned in July 2020 and designed to provide GMP manufacturing in compliance with US Food and Drug Administration (FDA) and European Medicines Agency (EMA) regulations and guidelines.
You May Also Like