Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
 Biopharmaceutical process-related impurities encompass all organic and inorganic materials that arise from the biomanufacturing process apart from the drug substance. According to ICH Q6B guidelines on test procedures and acceptance criteria for biotechnological products, these impurities include cell substrates such as host-cell proteins, host-cell DNA, endotoxins, and so on; inducers, antibiotics, media components, and chromatographic media used in purification processes; and solvents and buffer components (1). Impurities can cause undesired, deleterious pharmacological effects if ingested or injected by a patient, compromising product quality and patient safety.
Biopharmaceutical process-related impurities encompass all organic and inorganic materials that arise from the biomanufacturing process apart from the drug substance. According to ICH Q6B guidelines on test procedures and acceptance criteria for biotechnological products, these impurities include cell substrates such as host-cell proteins, host-cell DNA, endotoxins, and so on; inducers, antibiotics, media components, and chromatographic media used in purification processes; and solvents and buffer components (1). Impurities can cause undesired, deleterious pharmacological effects if ingested or injected by a patient, compromising product quality and patient safety.
Chromatographic purification of therapeutic proteins often begins with affinity chromatography to achieve high purity and recovery in a first step. Affinity binds only the protein of interest and thus removes many impurities including proteases. Often the affinity interaction incorporates a low-pH viral inactivation unit operation in the same step. One example uses pseudoaffinity interaction dye. Purification of some therapeutic proteins begins with Cibacron blue dye (HiMedia Laboratories) as a capture chromatography step. For example, recombinant human therapeutic hormones such as follicle-stimulating hormone (FSH) and human chorionic gonadotropin (HCG), both used for infertility treatment, use Cibacron Blue dye in their capture chromatography operation.
Such chromatography gels on the market include Blue-Sepharose 6 FF from Cytiva (formerly GE Healthcare). The dye is linked covalently to a Sepharose gel base matrix by the triazine binding method. Sepharose/cellulose base gels are white. According to ICH Q6B, the dye is a process-related impurity in the manufacturing of recombinant hormones. Thus, it is imperative to carry out an assessment of that dye in active pharmaceutical ingredient (API) drug substances before manufacturing of drug product proceeds and to estimate whether the dye’s presence is within acceptable limits. That holds true not only for the API, but also for the intermediary bulk or the in-process bulk material such as affinity elute.
Buffer Formulations |
|
Elute from the affinity step is loaded onto a polishing column, typically for ion-exchange chromatography (IEC): e.g., cation-exchange media such as carboxymethyl (CM) cellulose and anion-exchange media such as Cytiva Q Sepharose Fast Flow (Q-FF) gel and Bio-Rad Laboratories Macro-Prep High Q media, and diethylaminoethyl (DEAE) cellulose. By virtue of the ion-exchange mechanism, IEC operations are used to “polish” a protein of interest and remove remaining process- and product-related impurities after the affinity separation.
Elution from a Cibacron-based–gel chromatography step sometimes causes dye to leach with the protein of interest. Leached Cibacron blue dye can bind to ion-exchange gels too strongly to remove with any known buffer composition. The originally white gels slowly develop a bluish coloration. Over time, the gel becomes completely dark in the top layers of such an IEC column. This not only increases the possibility of dye leaching further into IEC elutes, but it also compromises the lifetime usability of the gel. In addition, blue coloration of the originally white gel often causes concern to operators. On repeated use of the subsequent gel over time, the coloration increases to such an extent that it becomes difficult to strip off the dye bound to the gel during cleaning-in-place (CIP). Such irreversible binding severely affects a gel’s quality and lifecycle, leading to its premature unusability for chromatography processes.
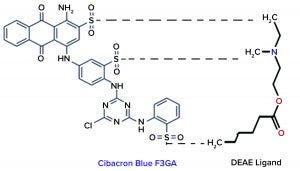
Figure 1: Interaction chemistry between Cibacron dye and DEAE ligand
Objective
Our company conducted a study for developing and optimizing an inorganic-solvent–based stripping buffer that can be used for removing the irreversible binding of Cibacron dye from IEC media, thereby restoring the gel properties for subsequent polishing chromatographic processes. We externally induced binding of Cibacron dye to an IEC gel — DEAE Sepharose 6 FF gel from Cytiva — and then stripped off the dye with a laboratory-made stripping buffer. Finally, we estimated the amount of dye stripped off with an in-house assay using a standard preparation of Hi-Cert–grade Cibacron blue F3GA dye powder (HiMedia) and spectrophotometric analysis at OD 610 nm.
Because Cibacron dye impurity comes from the blue Sepharose gel, we had established it as a process-related impurity in our clinical batches and from abnormal toxicity study results of our products. Based on the limits in those batches and assays, we analyzed process validation (PV) batches for the dye’s presence and quantity. Those batches demonstrated dye impurity levels well below the threshold of the test method — limit of detection (LoD) and limit of quantitation (LoQ) at 0.0005 ppm (0.5 ppb) — which is well in accordance with the clinical batches and toxicity assay materials. We also used this assay to estimate the amount of dye leached out in a pre-CIP cycle — in a protein-based in-process sample (nonbinding conditions) — and after a CIP cycle to determine whether all amounts fell within acceptable limits.
Methodology and Framework
Binding of Cibacron Dye to Ion-Exchange Gel: For binding, 5 ppm of Cibacron powder solution (HiMedia Cibacron blue F3GA; Hi-Cert) was prepared. The prepared solution was mixed with 25 mL of DEAE Sepharose gel and kept overnight at room temperature for binding. The next day, the gel was divided into 5-mL fractions and packed into separate XK 16/20 columns (Cytiva) at a bed height of 2.5 cm using an ÄKTA Prime Plus chromatographic system.
 After column packing, the gel was washed with 10 column volumes (CV) of purified water to remove unbound dye. Unbound dye in collected wash was analyzed spectrophotometrically. This was followed by two column washes of 5 CV each with purified water. The final 5-CV wash elute was collected and analyzed spectrophotometrically to confirm an absence of dye leaching from the gel. Afterward, the column was ready to use for the stripping experiment. Bound dye on the gel would be calculated from the difference in initial dye concentration used for binding to it and the concentration of unbound dye from the purified water washes in which dye had leached.
After column packing, the gel was washed with 10 column volumes (CV) of purified water to remove unbound dye. Unbound dye in collected wash was analyzed spectrophotometrically. This was followed by two column washes of 5 CV each with purified water. The final 5-CV wash elute was collected and analyzed spectrophotometrically to confirm an absence of dye leaching from the gel. Afterward, the column was ready to use for the stripping experiment. Bound dye on the gel would be calculated from the difference in initial dye concentration used for binding to it and the concentration of unbound dye from the purified water washes in which dye had leached.
Stripping Buffer Development and Optimization: As Figure 1 shows, ionic interactions occur between the sulphate groups of Cibacron dye and amino groups of DEAE ligand. Based on that interaction, the dye’s cyclic rings show an affinity toward the Sepharose matrix of IEC media, which is irreversible and accumulates over time, with blue-dye leaching affecting the IEC gel’s binding capacity. We considered using a high-molarity salt under acidic conditions to disrupt that ionic interaction, with the use of an organic solvent to disrupt aromatic interactions between the multicyclic dye and gel matrix.
Based on that postulation — and depending on different parameters such as ionic strength, polarity, pH ranges, and so on — we prepared five solutions (see the “Buffer Formulations” box) considering the chemistries of Cibacron dye and ligand on the DEAE Sepharose gel. The components used for making the buffers were salt, normal phosphate, a weak acid, and an inorganic solvent such as isopropyl alcohol (IPA). We did not use a strong acid or base because such harsh components would tend to leach the ligand from the DEAE Sepharose media.
We prepared 100 mL of each solution and passed that through the 5-mL Cibacron-dye–coupled DEAE gel columns individually for 10 CV (50 mL) at a residence time of eight minutes.
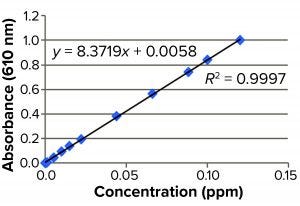
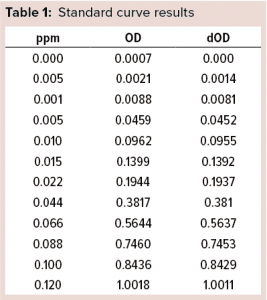
Figure 2: Standard curve for blue dye
Spectrophotometric Estimation of Cibacron Blue Dye: We used a spectrophotometric assay to estimate the quantity of Cibracron dye stripped off into each individual elute obtained from the columns. For a standard preparation, 8.4 mg Cibacron Blue F3GA dye powder is dissolved in 10 mL of purified water. A standard curve is constructed in the range of 0.0005–0.1200 ppm, which gave the LoQ and LoD of 0.0005 ppm (0.5 ppb) for the assay.
The assay is performed by measuring spectrophotometric absorbance of that solution at 610 nm using a Jasco V550 UV-vis spectrophotometer, then estimating the percentage of dye recovered from each elute. After noting which elutes showed higher dye recovery, we further optimized the buffer for enhanced stripping of dye from the IEC gel. Then we used the assay to estimate the amount of dye leached out in the pre-CIP cycle, in a protein-based in-process sample (under nonbinding conditions), and from a post-CIP cycle to see whether they fell within acceptable limits based on our clinical batches and toxicity studies. Finally, we carried out a spike recovery of Cibacron dye from in-process sample buffer to verify suitability of assay.
Results and Discussion
Stripping Buffer Evaluation: Table 1 and Figure 2 detail our preparation of a standard curve. The prepared stock consisted of 8.4 mg of Cibacron blue F3GA added to 10 mL of purified water as a diluent.
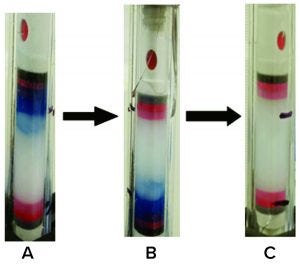
Figure 3: Removal of Cibacron Blue dye using 2M NaCl + 30% IPA at pH 3.0
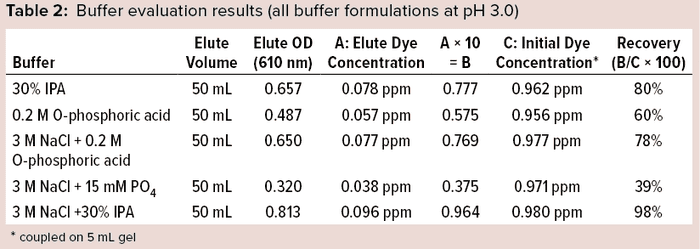
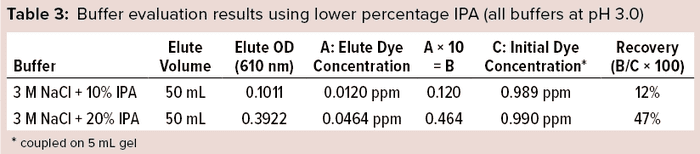
Preliminary studies gave the best results with a solution of 3 M NaCl + 30% IPA at pH 3.0 (Table 2). Based on the chemistry and interaction of the Cibacron dye aromatic-ring structure with the DEAE ligand, we conducted experiments using a lower percentage of IPA with 3 M NaCl salt molarity under acidic conditions independently to determine the effectiveness of dye removal at a lower IPA percentage. As Table 3 shows, both solutions with less IPA showed lower recovery than that using 30% IPA + 3 M NaCl, which showed consistently high recovery (~98%).
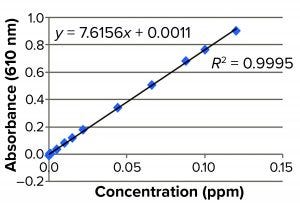
Figure 4: Standard curve
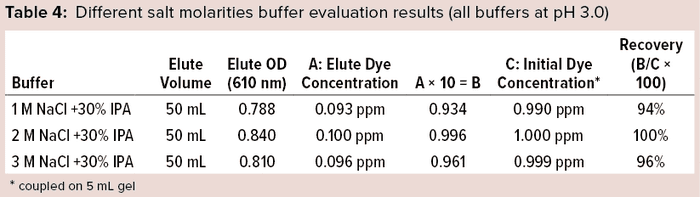
We further enhanced the 3M NaCl +30% IPA (pH 3.0) buffer after finding it to be heterogenous, with dissolution issues even after sonication. We prepared different molarities of the salt with the same percentage of IPA (30%) and pH (3.0) and evaluated them for Cibacron dye recovery (Table 4). The final stripping buffer solution was 2M NaCl + 30% IPA at pH 3.0, which showed homogeneity and complete removal of coupled dye from the DEAE gel (Figure 3).
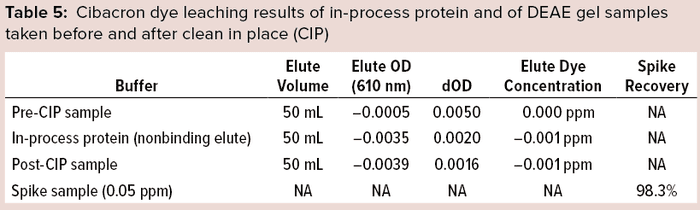
Spectrophotometric Results: We analyzed pre-CIP material, an in-process protein sample, and post-CIP samples of DEAE gel spectrophotometrically for leached Cibacron dye at absorbance 610 nm. Method suitability was demonstrated with spike-recovery of a known concentration of dye solution from diluent buffer.
The prepared stock consisted of 8.4 mg of Cibacron blue F3GA added to 10 mL of phosphate buffer. We used 15 mM PO4 + 0.3 M NaCl Buffer at pH 8.0 as a diluent and standard curve preparation as shown in Figure 4. No DEAE gel samples were found to have Cibacron blue dye present to any level below the method LoD (<0.0005 ppm or 0.5 ppb). The method suitability of this assay also passed, with 98.3% recovery of the dye (Table 5).
Our in-house–developed stripping buffer effectively removes 100% of bluish coloration associated with Cibacron dye from affected IEC DEAE gels. As evident from our spectrophotometric results, the coloration is removed from the gel by passing at least 10 CV of stripping buffer through it. Thus, we conclude that using this stripping buffer for 10 CV during pre-CIP and post-CIP cycles of IEC chromatography columns that follow the blue gel affinity process will effectively remove the bluish coloration associated with the dye binding. That will prevent damage of the IEC gel over time and thus help to increase the lifecycle of the media.
These results show that Cibacron dye removed by the stripping buffer is undetectable or negligible in the pre-CIP, in-process bulk, and post-CIP samples and therefore falls within acceptable limits to comply with our clinical-batch and toxicity-study criteria. We recommend using such a stripping buffer for 10 CV during the pre-CIP of IEC chromatography processes that follow blue-affinity chromatography and then checking in-process or final API samples for leached dye using a spectrophotometric assay.
Reference
1 ICH Q6B. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. US Fed. Reg. 64, August 1999: 44928; https://database.ich.org/sites/default/files/Q6B%20Guideline.pdf.
Further Reading
Crowley TE, Kyte J. Purification and Characterization of Ferredoxin-NADP+ Reductase from Chloroplasts of S. oleracea. Experiments in the Purification and Characterization of Enzymes. Academic Press: Cambridge, MA, 2014; 25–102; https://doi.org/10.1016/B978-0-12-409544-1.00001-1.
Curling J. Affinity Chromatography: From Textile Dyes to Synthetic Ligands By Design, Part I. BioPharm Int. 17(7) 2004; https://www.biopharminternational.com/view/affinity-chromatography-textile-dyes-synthetic-ligands-design-part-i.
HiTrap DEAE Sepharose FF. Cytiva: Marlborough, MA, 2020; https://www.cytivalifesciences.com/en/us/shop/chromatography/prepacked-columns/ion-exchange/hitrap-deae-sepharose-ff-p-00605#tech-spec-table.
Corresponding author Pranav Milind Gupte is assistant manager, Mahesh Gavasane is deputy general manager, Tushar Bhabal is executive, and Saher Cheulkar is officer of biotech R&D in recombinant biotherapeutics downstream processing and formulation development at Bharat Serums and Vaccines Limited, Third Floor, Liberty Tower, Plot No. K-10, Behind Reliable Plaza, Kalwa Industrial Estate, Airoli, Navi Mumbai – 400 708, India; 022-45043100; [email protected]. ÄKTA Prime Plus, Blue Sepharose FF, DEAE Sepharose 6 FF, and XK 16/20 are registered trademarks of Cytiva (formerly GE Healthcare). V550 UV-Vis is a registered trademark of Jasco.
You May Also Like