Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Chromatographic purification remains the most critical step in biopharmaceutical downstream processing. Its purpose is to separate biologic impurities such as host-cell proteins (HCPs), nucleic acids, and oligomers from a target biologic, which must be purified to very high levels (often >99%). Biological separations usually require medium to high salt concentrations and bear inherent risks of microbial contamination in waterbased process streams. Thus they require specifically designed equipment. Depending on process constraints, chromatographic media, and equipment limitations, biochromatographic separations may be run at low to very high velocities (e.g., 75 to >500 cm/h).
Photos 1:

Photos 1: ()
Columns are the most critical part of biochromatography systems because they are where the separation takes place. When choosing a column, several points should be taken into account, depending on process constraints and plant design: scale of operation (current and projected), whether it occurs in a dedicated or a multipurpose plant, chromatography media lifetime (frequency of unpacking/repacking operations), the maximum allowed process cycle times, process step(s), type(s) of media, and of course the recovery yield of target product (purity is not negotiable). Evaluation and rating of those parameters will help direct the choice of column technology: design, materials of construction, performance, and the degree of automation and handling. Although disposable chromatography columns may be valuable alternatives to traditional equipment for certain cases, their performance is intrinsically limited, and their utility may be limited by substantially higher external costs compared with reusable columns. We wish to highlight the special features of an industrial biochromatography column designed to meet some of the industry’s challenging needs, such as
operations in multipurpose biomanufacturing plants (such as contract manufacturers), which need to reduce operating costs or face production bottlenecks
requirements to pack large (>1 m I.D.) columns right the first time
cycle time optimizations for purification requiring frequent packing and unpacking operations
high efficiencies needed for optimizing the yield of difficult separations during purification and polishing steps (for heparins, coagulation factors, albumin GPC steps, hydroxyapatite steps, and so on).
PRODUCT FOCUS: ALL PROTEIN BIOPHARMACEUTICALS
PROCESS FOCUS: DOWNSTREAM PROCESSING
WHO SHOULD READ: PROCESS DEVELOPMENT AND MANUFACTURING
KEYWORDS: COLUMN PACKING, REPRODUCIBILITY, SCALE-UP
LEVEL: INTERMEDIATE
Photo 2:

Photo 2: ()
Desirable Features
Other than sanitary design and material compatibility, several column design options are available for CGMP production. End users are looking for cost-effective solutions that provide the highest yield and purity with these features: reproducibility, scalability, speed, ease of use, safe handling, versatility and equipment robustness.
Here we present data that will demonstrate that Prochrom-Bio lowpressure chromatography columns equipped with a hydraulically actuated piston meet all those requirements for a cost-effective solution. These columns have been tested and packed with most commonly used gels, showing excellent performance whatever the column diameter. The most important design features of Prochrom-Bio columns are the presence of a hydraulically operated piston and specially designed packing/unpacking valves located at the bottom of each column barrel. The piston’s purpose is not to keep the bed compressed (which is the case for high-pressure dynamic axial compression columns used with rigid media) but merely for quickly, conveniently, and reproducibly packing and unpacking the column and to adjust its bed height optimally to a desired value.
Packing recipes developed by Novasep process engineers are integrated into Novasep proprietary software installed in the system’s packing and unpacking stations, which makes the process completely automated and fully closed. It features slurry introduction at high velocity through the packing valves for uniform bed-packing results. Packing time is typically
Reproducibility is a key element to consider when packing a biochromatography column. Indeed, similar performance (measured using the number of theoretical plates and peak asymmetry) should be obtained each time the column is packed, provided that both the resin and procedure are identical, for the process to be reproducible from batch to batch. Prochrom-Bio columns allow end users to obtain similar performance with repeated packings.
We tested a Prochrom-Bio LP 150mm I.D. column with DEAE-650M grade Toyopearl gel from Tosoh Bioscience (www.separations.us.tosohbioscience.com) (Figure 1). Our results showed 4,000 ±500 plates/m at 150 cm/h (an average over 20 successive packings), with a peak asymmetry of 1.0 ± 0.2. We used NaCl 0.15 M in osmosed water as an eluent and an injection of 2% acetone in 0.15 M NaCl (2% of CV).
Scalability is also critical. During industrialization of an optimized process, results obtained for a small column diameter should be extrapolated to a larger one and vice versa. As a result, the packing step should generate the same performance no matter the column diameter. As Table 1 shows, this is indeed the case. We obtained those results using Fractogel EMD DEAE-M resin from EMD Chemicals Inc. (www.merck-chemicals.com) in columns with widely different internal diameters (150 mm, 300 mm, and 1,200 mm). Eluent velocity was 75 cm/h. Actually, slightly better efficiencies are often obtained with larger-diameter Prochrom-Bio columns due to reduction of the ratio between column wall length and cross section — and thanks to optimized flow distribution technology. Scale-up is guaranteed for the packing step using Prochrom-Bio columns with automated packing methods.
Table 1: Scalability with Prochrom-Bio columns

Table 1: Scalability with Prochrom-Bio columns ()
Speed: With increasing upstream titers, downstream processing is now considered the bottleneck in biopharmaceutical production. To address the bottleneck, process engineers continually look to speed up their chromatography step(s) by reducing downtime and decreasing the time needed for packing and unpacking columns. With a Prochrom-Bio system, the column maintenance time is minimized. It is typical to unpack and repack a Prochrom-Bio column in
Operating speed is directly related to how easy equipment is to handle. The overall design of a Prochrom-Bio column (particularly the design of its piston and packing/unpacking valves) allows for easy maintenance. The piston is controlled by a hydraulic unit requiring only compressed air, which operates a hydraulic jack to which the piston is attached by a clamp. The chromatographic bed cannot be contaminated. Packing (and unpacking) recipes are stored in the packing and unpacking station’s software, which is 21 CFR part 11 compliant. An operator selects the most suitable method considering his or her specific conditions. In addition, operator safety is ensured because no operations involve manipulating heavy parts; and the media, buffers, and process fluids are contained at all times. Novasep’s proprietary unpacking procedure allows gel to be removed using only a small amount of buffer (two to three CVs), which enables quick and easy repacking of gel in a Prochrom-Bio column.
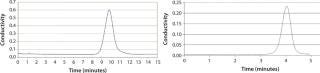
Figure 3: left: 15-cm bed height, 1.07 asymmetry, N = 562 plates, N/L = 3,750 plates/m, 90 cm/h velocity, 60–90 µm particle size, 0.83 total porosity, 30-mL 3 M NaCl injection, 300-mm column diameter
right: ()
These columns represent a solution of choice for all gels available on the market and provide optimal results with numerous media (Figure 1, Table 2). Versatility is thus guaranteed. The table summarizes some results obtained using different media and column sizes. The “Time” column indicates packing operation duration for each.
Table 2: Experimental data generated with Novasep packing recipes

Table 2: Experimental data generated with Novasep packing recipes ()
A Versatile Alternative
Downstream processing often is and will remain a bottleneck in most biomanufacturing plants. Although disposable chromatography columns are a well-advertized option, they are not the most cost-effective solution for every plant — in particular, not when production requires frequent unpacking and repacking operations.
Prochrom-Bio low-pressure chromatography columns address the criteria that end users are looking for in numerous challenging pilot and industrial-scale biochromatography processes. The columns are safe and easy to handle, and their design promotes optimal performance with any resin at any scale.
About the Author
Author Details
Jonathan Albanese is ADC production supervisor at Novasep Synthesis (Le Mans); Jean Guillerm is biopharma process expert at Novasep Process (Pompey); Henri Colin is a consultant for Ulysse Consult (Nancy); Jean Blehaut is director of marketing and business development at Novasep; and corresponding author Hélène Chochois is marketing manager for Groupe Novasep Biomolecules, 82 boulevarde de la Moselle, 54340 Pompey, France; 33-(3)-8349-7147, fax 33-(3)-8349-7149; [email protected].
1.) Bloominburg, G, and P. Gandhi. 2005. Engineering Design Considerations for Column Packing in Large-Scale Biotechnology Facilities. BioProcess Int. 3:44-51.
2.) Bemberis, I, A Noyes, and V. Natarajan. 2003. Column Packing for ProcessScale Chromatography: Guidelines for Reproducibility. BioPharm Int..
3.) Jullien, S, J Albanese, and J. Guillerm. 2008–2009. Novasep Packing Studies.
You May Also Like