Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
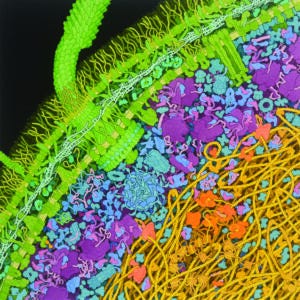
Depicted here as a lattice of proteins between the inner and outer membranes of an Escherichia coli cell, peptidoglycans and flagellar filaments are known to generate significant immune responses to biological drug products. ADAPTED FROM IMAGE BY DAVID GOODSELL, HTTPS://COMMONS.WIKIMEDIA.ORG/WIKI/ FILE:ESCHERICHIA-COLI-BACTERIUM(1).TIF
Process-related impurities such as host cell proteins (HCPs) can raise concerns about biological product efficacy, quality, safety depending on their properties and levels. In the first part of this series, we surveyed relevant regulatory frameworks and detailed potential effects of HCPs on biologic efficacy. Here in part 2, we review available literature on HCPs and patient safety, including information about HCP-related immune responses and adverse clinical events.
HCP Effects on Patient Safety
At least five HCP-induced factors can influence a therapeutic protein’s
safety profile:
• immunogenicity — activation of innate or induction of adaptive immunological reactions at the site of injection, formation of binding and/or neutralizing antibodies (IgM and IgG, respectively), and release of proinflammatory cytokines (e.g., tumor necrosis factor alpha, TNFα)
• allergenicity — early phase allergic reactions related to IgE induction (e.g., anaphylaxis) and IgE-independent reactions (e.g., late-phase reactions related to cytotoxic T cell CD8+ induction) with the consequence of tissue damage
• potential for interaction or binding with therapeutic proteins (folded/unfolded/aggregated), with induction of binding and neutralizing antidrug antibodies (ADAs) related to adjuvant effect
• potential for aggregation related to repeating patterns, which can activate marginal-zone B cell (MZB) activation
• potential for biological activity — e.g., monocyte chemoattractant protein 1 (MCP-1) and transforming growth factor beta 1 (TGFβ-1).
HCP-Induced Immune Responses
By themselves, HCPs can generate extreme immune responses. Of particular concern are bacterial HCPs such as flagellin (35) and peptidoglycan. It is unsurprising that HCPs from Escherichia coli can elicit immediate immune responses considering that the species is part of our microbiota (36) and is widespread throughout the environments surrounding human populations.
Strong and Sustained Immune Responses: To understand how HCPs induce immune responses, consider biologics that are designed to induce sustained and strong immune responses: adjuvanted vaccines. Without adjuvants, purified antigens (Ags) do not result in effective, durable immunization (37, 38). Purified Ags usually are mixed with hydroxide or phosphate aluminum, squalene, CpG class B nonmethylated DNA, or lipid A monophosphoryl (MPL) to induce the expected response. Other adjuvants are under investigation (39, 40).
Aluminium hydroxide, Al(OH)3, is an excellent example to demonstrate how adjuvants induce immune responses. It manifests as an aggregation of micrometric particles (41). Macrophages confuse these microparticles with needle-shaped bacteria (2–10 µm) and phagocytize them. When vaccines are injected subcutaneously, macrophages migrate to the site of puncture and engulf aluminum-Ag particles, then persist for months at the injection site. Studies also have demonstrated that macrophages retain aluminum deposits in their lysosomes after vaccine injections (42–44). Aluminum-bearing myeloid cell lines and antigen presenting cells (APCs) differentiate into mature CD83+ dendritic cells (DCs) with typical morphology. That process is induced by autocrine secretion of interleukin (IL) 4 and 12 and by paracrine secretion from regulatory T (Treg) cells (45).
Aluminum activates macrophages by causing cell necrosis, activating cathepsin B in lysosomes and, in turn, inducing inflammasome assembly (see 46 for an in-depth review; 47). The inflammasome recruits procaspase-1, which oligomerizes and undergoes autoproteolysis. The resulting caspase-1 digests pro-IL-1b and pro IL-18. Then the activated ILs promote inflammation and adaptive immune responses from type 2 helper T (Th2) cells (48, 49).
This sequence of events recruits more T cells, DCs, basophils, and mast cells to the site of trauma. Basophil- and mast-cell degranulation increases cellular permeability, diapedesis, and secretion of vasoactive amines (50). By themselves, APCs secrete ILs such as prostaglandin E2 (PGE2), which induces lymphocyte T CD4+ differentiation and DC activation (51, 52). Some T cells become memory cells, which provide long-lasting immunization for some Ags (53).
Other vaccines contain live and/or attenuated viral or bacterial components processed with denaturing agents such as formaldehyde (see 54 for an extended review). Attenuated vaccines can contain single- or double-stranded RNA, nonmethylated CpG DNA, or glycoproteins from a target virus (55, 56) or flagellin, pilin proteins, peptidoglycan, outer membrane porin (Omp)C, OmpF, and lipopolysaccharides (LPSs) from bacteria.
Such molecules are strong inducers of immune responses (57). Pathogen-associated molecular patterns (PAMPs) induce stress signals at the site of injection (through keratinocytes, fibroblasts, or APCs) after having been phagocytized by APCs. After detection by pattern-recognition receptors (PRRs), APCs secrete interferons (IFs) (58) and recruit by chemiotaxis more immune cells to the site (59). PRRs related to innate immune responses include
• C-type lectin receptors
• nucleotide-binding and oligomerization domain-like receptors (NODLs)
• gxv retinoic-acid–inducible gene-I–like receptors
• toll-like receptors (TLRs).
The latter receptors can trigger strong immune responses quickly (54). For example, TLR4 recognizes bacterial LPS, triggering transduction signals that figure into the inflammasome machinery assembly inside immune cells (46), whereas in mice TLR11 can detect flagellin and profilin-like proteins (60).
In summary, to elicit an immune response, APCs must phagocytize HCPs, then process and present their Ags to major histocompatibility complex class II (MHC II) molecules (or to the human leukocyte antigen (HLA) system) after stress promoted by an agent such as cathepsin B, uric acid release, or other mediators of immune responses.
Live–attenuated vaccine drug products are not manufactured with adjuvant molecules (e.g., aluminum) or other substances that enhance immune responses. United States Pharmacopeia 39–National Formulary 34 chapter <1132> on “Residual Host Cell Proteins Measurement in Biopharmaceuticals” states that immunization of animals with humanized HCPs must be executed with the appropriate adjuvant, which can be incomplete or complete Freund’s adjuvant (61), to elicit an adequate immune response.
Moreover, the dose for a strong response must be between 5 µg and
50 µg per dose, with the adjuvant present (61–69). Concentrations below that range can cause anergy, in which responsive T cells are depleted, inducing tolerance (70–73). During that process, clonal expansion is inhibited due to the absence of danger-associated molecular patterns (DAMPs) or a naturally occurring infection.
Some Ags cannot elicit a sustained immune response (and therefore inflammatory and/or allergic responses) at doses of 7.5 µg without adding an adjuvant to the pharmaceutical formula (74). Otherwise, the Ags are considered to be insufficiently antigenic (62).
Perhaps — and this is an opinion — the absence of PAMPs and DAMPs is the reason that some drug products have shown only the presence of binding antibodies. That also might explain why some products generate T-cell–dependent responses (mainly IgG responses) or T-cell–independent (IgM) responses but no neutralizing antibodies. Can HCPs function as inducers? That seems to be dependent on their nature.
It is worth mentioning that all reports of HCP-related immune reactions come from E. coli–derived products manufactured using legacy generation purification processes (18, 19, 75). That expression system produces all of the aforementioned molecular signals that can elicit strong immune responses. Studies indicate that E. coli HCPs can induce an immunological response (e.g., to CpG DNA, formyl methionyl-proteins, LPS, peptidoglycan, and flagellin) through TLRs (76). Moreover, because overexpressed proteins form inclusion bodies, impurities from such particles easily can remain in a drug substance/product after purification. Indeed, purification processes are not HCP-proof.
Immunogenicity Related to Small Protein Variations: Available data contradict Janeway’s theory that pure proteins do not induce immune responses in the absence of an adjuvant (77). Recombinant therapeutic proteins can be immunogenic if they are nonidentical to native proteins.
Early in the history of immunotherapy, murine monoclonal antibodies (MAbs) were designed for cancer treatment. The US Food and Drug Administration (FDA) approved the first such product, Orthoclone OKT3 (muromonab-CD3, Janssen-Cilag), in 1986 for treatment of acute allograft rejection. The MAb was associated with adverse hypersensitivity effects related to the development of ADAs. The murine origin of the construct elicited the immune response (78, 79). Therefore, a humanized version of the anti-CD3 murine antibody (Ab) was developed using a routine hybridoma technique. The new construct, 12F6, proved to be less immunogenic than its original counterpart (78).
Subsequently, researchers transitioned to developing chimeric, humanized, and fully humanized MAbs. Some of the resulting products retained their immunogenic characteristics (78, 80) even if they were completely humanized MAbs (81). But not all therapeutics have encountered such difficulties. Amgen’s Enbrel (etanercept), a fusion protein of TNF receptor 2 (TNFR2) and its Fc domain, does not induce immunogenicity (82). An explanation for that outcome is that both domains are 100% human. Although later examples present contradictory data, MAb immunogenicity seems to be related to an Ab’s complementarity-determining region (CDR), which induces anti-idiotypic antibodies (82) or proteins with nonnative epitopes (18, 19, 84).
Such examples raise serious concerns for therapeutic MAbs. However, immunogenicity to foreign proteins (e.g., coming from E. coli, yeast, or nonhuman mammalian cells) seems not to be a problem unless it elicits extreme inflammatory or IgE responses. Additionally, studies of immunogenicity report that aggregation is necessary to induce such responses (77) and that the presence of inductors (40) or “superantigens” such as LPS (85, 86) can be highly immunogenic.
Research and development (R&D) departments all around the world have worked to overcome such issues. Current purification processes can reduce critical impurities to very low levels. However, questions remain:
• Can formation of HCP–therapeutic protein complexes accelerate immunogenic responses?
• Can strong immune responses related to injection of PAMP, DAMP, and alarmin impurities mixed with a therapeutic protein elicit cross-reactive responses?
Answers to such questions have been elusive.
Adverse Reactions Related to IgE Induction and Anaphylactic Responses: Systemic allergic reactions are expected. HCPs and therapeutic proteins are not endogenous and, therefore, can induce hypertension, edema, fatigue, asthenia, dyspnea, headache, nausea, vomiting, chest pain, skin reactions, seizures, and other allergic reactions (87–90).
Some clinical studies for Epogen (epoetin alfa, Amgen) have shown that a placebo induced a higher percentage of injection-site allergic reactions (12%, subcutaneous) than did the reference product (7%) (87). It is important to mention that some of those reactions were associated with the therapeutic proteins themselves rather than an impurity present in a drug product (89).
Nevertheless, biological products can cause adverse events such as anaphylaxis, which accounts for 500–1,000 deaths each year in the United States (91), an estimated 0.33 deaths/year/million people in the United Kingdom (92), and about 0.66 deaths/year/million people in Australia (93, 94). Among the main causes of anaphylactoid fatal responses are medication (44%), food (31%), and insect stings (23%) (95, 96). Thus, it is critical to measure HCP levels in biopharmaceutical drug products.
Ags must have specific characteristics to be considered as allergens. They must:
• be stable proteins
• be enzymatically active (e.g., protease activity)
• induce responses at low doses (e.g., IL-4 inductor)
• have high solubility
• have low molecular weights
• interact efficiently with MHC II/HLA systems (see Figure 12.3 in 77).
Many allergens must be inhaled to cause a reaction; however, certain proteases in food can become allergenic in the intestines. Proteases in fecal pellets of house dust mites can induce IgE-specific signals with related allergy reactions (see Figure 12.5 in 77). In mice, injected active proteases such as papain can trigger IgE responses (97). And allergens can become occupational hazards for food-industry employees who work with meat tenderizers containing bromelain and papain (98, 99).
Allergies can be divided into four types based on their immune-reaction pathways:
• type 1 — mediated by IgE activation of mast cells
• type 2 — mediated by IgG antibodies against cell surfaces and matrices
• type 3 — mediated by Ag–Ab complexes
• type 4 — mediated by T cells.
Anaphylaxis is an exacerbated type 1 response. Ags travel in low concentrations through some route (e.g., orally or through mucus membranes in the cases of food and small-molecule pharmaceuticals) and are digested by APCs. Then, they are presented through the MHC II to Treg cells, inducing expression of a Th2 phenotype and secretion of IL-4, IL-13, and CD154 after activation (48, 77). In B cells, the initial stimulus triggers a switch from IgG to IgE production. Abs released by plasmatic cells (differentiated B cells) bind to high-affinity IgE receptors (FcεRIs) on mast cells and basophils (50), which then secrete mediators that are responsible for the classic asthmatic and anaphylactoid profiles with corresponding vasodilation or bronchoconstriction depending on the affected organs (100). In the “classical anaphylactic pathway,” IL-13 and IL-33 also are secreted by type 2 innate lymphoid cells (ILC2s) (101). These secreted mediators — namely histamine (major mediator), IL-4 (secreted by basophils), leukotrienes, prostaglandins, β-tryptase, platelet activator factor, cytokines, and chemokines — are the main mediators of IgE immune responses (50, 102).
Adverse reactions related to anaphylaxis include cellular permeability, with movement of interstitial fluid to extracellular compartments. Such movement can induce hypovolemic shock (103) with associated dyspnea, itching, hypotension, abdominal pain, syncope, bronchospasm, and ultimately, even heart failure and death (104).
The FDA has reported allergic reactions related to small-molecule drugs such as chlorhexidine gluconate (105) and asenapine maleate (106). The agency also has issued warnings about small proteins such as recombinant human growth hormone (rhGH):
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products. (106)
About Fiasp (fast-acting insulin aspart, Novo Nordisk), the agency notes
Severe, life-threatening, generalized allergy, including anaphylaxis, generalized skin reactions, angioedema, bronchospasm, hypotension, and shock may occur with any insulin, including Fiasp, and may be life threatening. In the clinical program, generalized hypersensitivity reactions (manifested by generalized skin rash and facial edema) were reported in 0.4% of adult patients treated with Fiasp. Allergic skin manifestations reported with Fiasp in 1.7% of adult patients from the clinical program include eczema, rash, rash pruritic, urticaria and dermatitis. (107)
Infliximab administration can generate IgE and IgM ADAs. Adverse effects were rare in one study, occurring in only three of 71 subjects (108). Such reactions stemmed from the product’s chimeric origin. In another study, however, 61% of subjects developed immunogenicity to the product (109).
Xue et al. have detected Abs against HCPs from an infliximab Chinese hamster ovary (CHO) cell line in subjects who had no known exposure to biologics (110). Among those Abs, IgG1 and IgG2 subclasses predominated, appearing in 67% and 60% of study participants, respectively. Additionally, 40% of subjects were reactive to IgM subclass Abs but showed no significant IgE activity. Three patients reported severe reactions due to anti-infliximab IgEs and skin reactions.
A study by Ingerslev et al. indicates that Abs against CHO HCPs were present in hemophilia patients before and after treatment with recombinant human blood factor VIII (rhFVIII), although such Abs were responsible for none of the adverse events reported (111). Anti-rhFVIII Abs, however, can neutralize the therapeutic, becoming a serious quality issue.
Thus far, all reports of HCP-related anaphylaxsis have involved aggregated proteins.
Can HCP Interaction with Therapeutic Proteins Induce ADA Production? Another important risk factor is potential for generating ADAs, which can be binding or neutralizing Abs.
HCPs can coelute with therapeutic proteins when they exhibit similar physicochemical properties (e.g., a similar isoelectric point, pI). HCPs can establish Van der Waals interactions with hydrophobic patches on the surfaces of drug substances. That process can induce exposition of reactive (immunogenic) epitopes (112).
Therapeutic protein complexes can direct cross-reactive antibodies against HCPs. Such cross-reactions can mediate an autoimmune process in which levels of endogenous proteins can be depleted. For instance, erythropoietin depletion can result in a severe illness called pure red cell aplasia (PRCA), an autoimmune disease characterized by severe anemia (113, 114), absence of red blood cell precursors from bone (<109/L), erythropoietin nonresponsiveness, and formation of ADAs that also neutralize endogenous erythropoietin (115, 116).
Between 1998 and 2002, 12 severe Ab-mediated PRCA cases occurred, 11 of which involved subcutaneous administration of Eprex (epoetin alfa, Johnson & Johnson), a serum-albumin–free formulation of erythropoietin. Those cases were linked to leachates from uncoated rubber plunger stoppers in prefilled syringes. The leachates generated an emulsion that created erythropoietin aggregates, and subcutaneous injection induced the autoimmune illness (117, 118). After the stoppers were coated with polytetrafluoroethylene, the incidence of PRCA diminished by ~90% (116). This example demonstrates that interactions involving leachates, emulsions, and aggregates can induce an autoimmune disease (118, 119).
A 2017 study measured ADAs in 128 patients treated with recombinant human erythropoietin (rhEPO) (120). Only three subjects showed correlation between anemia and ADAs. The available data show some factors that can trigger ADAs against rhEPO, including micelle formation, leachate release, presence of glycine in the formulation, and subcutaneous injection, which is related to PRCA events in patients treated with erythropoietin.
Experiments on induction of immune response in vaccines indicate that to elicit a strong response against a protein (e.g., a therapeutic), the formulation must include LPS (62–65, 75); its detoxified counterpart, monophosphoryl lipid A (MPL) (121); or at least a suitable adjuvant.
Many biotechnological products have been sold since the 1980s, making a great amount of data available over the years since then. It can be consulted any time using the websites of regulatory agencies. Following distribution of a therapeutic product, strict pharmacovigilance protocols can be an advantage for early detection of adverse effects, such as those stemming from variability across biotherapeutic batches, idiosyncratic patient reactions, and differences across patient subpopulations (e.g., ethnicity and other factors not assessed in clinical trials).
In clinical facilities, “allergenic responsiveness tests” can prevent the worst IgE-mediated and anaphylactoid responses (122). Practitioners can perform such tests using subcutaneous punctures for fast IgE responses or slow T-cell (CD8+) responsiveness due to increase of antigen threshold (123). Early anaphylactoid tracking can help to determine responsiveness to therapeutic proteins and to trace HCPs, DNA, or RNA present in drug product.
Notably, there is a lack of research into whether bacterial proteins — or those from other expression systems — can trigger ADAs in response to direct interaction with therapeutic proteins. However, researchers have linked ADAs to losses in drug-product efficacy resulting from complexing of HCPs and therapeutic proteins.
Biological Activity: A study by Beatson et al. demonstrates constitutive expression of TGFβ-1 in CHO-K1 cells (124). The protein shows biological activity at low concentrations. In the study, it also coeluted with a fusion protein composed of a mouse IgG2 Fc region fused to mucin 1 (MUC1) ectodomain, indicating that both proteins have similar pI (~5.5). TGFβ-1 was present in a ratio of 19.4 ng for every milligram of therapeutic protein. The team also found that the immunomodulatory effect of MUC1 could be attributed, at least in part, to a cytokine impurity found in the samples and that the effect can be inhibited using neutralizing Abs against TGFβ-1.
Such activity was unexpected for an HCP. Cultivation media were tested for TGFβ-1, with negative results returned by an enzyme-linked immunosorbent assay (ELISA) kit. Using reverse-transcriptase polymerase chain reaction (RT-PCR), the team found the cytokine gene in the expression system. They ultimately determined that TGFβ-1 derived from the host cells and that it was present in the supernatant as part of a large latent complex (LTBPx-LAP-TGFβ-1) of 213–275 kD, as demonstrated by ultrafiltration (100 kD) (124). Of particular interest is that despite being of hamster origin, the cytokine is biologically active in human cells, as determined by a plasminogen activator inhibitor 1 promoter–luciferase assay using mink lung epithelial cells (Mink-PAI-1-Luc) (125).
Although Beatson et al. report that the identified cytokine is active only after incubation at 80 °C for 10 minutes, another study found increased TGFβ-1 levels in the blood of patients who received intravenous immunoglobulins (126). Subsequent research identified several activation mechanisms such as mild mechanical agitation, protease cleavage, or pH stress in vivo (127). It is worth mentioning that almost all MAbs and glycosylated therapeutic proteins are expressed in CHO cells (128). Thus, HCP-TGFβ-1 activity must be tracked for all patients treated with such products until the problematic gene can be deleted from CHO cell expression systems or until purification processes can remove the impurity to very low levels. The molecule exerts important biological functions such as G1 cell arrest.
In another well-conducted study, Haggerty et al. described the presence of the host-cell–derived chemokine MCP-1 (129). The impurity can induce release of histamine by basophils. In phase 2a clinical trials, patients presented serious adverse events relating to histamine release after treatment with Orencia (abatacept, Bristol Myers Squibb). Using an in vitro assay of whole blood, the researchers detected the presence of released histamine. Identification of the impurity was performed by reversed-phase liquid chromatography with tandem mass spectrometry (RP-LC-MS/MS). The HCP showed high homology to human MCP-1.
Ensuring Drug Product Safety
The examples herein point out that rigorous physicochemical analyses, impurity quantification, bioanalyses, clinical trials, and pharmacovigilance must be performed to ensure drug product safety in the short and long terms during commercialization. New information and improvements to in vitro assays could shed light on and enhance detection of now-undetectable safety issues.
The next installment of our review, which will appear in BPI’s November–December 2022 issue, provides case studies in HCP identification. The series will conclude in January–February 2023 with discussion of best practices for HCP risk management and exploration of the future of HCP analytics.
Acknowledgments
This work was supported by Mexico’s Consejo Nacional de Ciencia y Tecnología (CONACyT), Grant FINNOVA-CONACyT 174104. Authors VPMM and NOP are Sistema Nacional de Investigadores (SNI)-L1 CONACyT Fellows.
Disclosures
Probiomed S.A. de C.V. develops, manufactures, and markets biosimilar products. All three authors are involved in the development of biosimilar products for Probiomed.
References
See part 1 for references 1–17, 20–34.
18 Wadhwa M, et al. Immunogenicity of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Products in Patients Undergoing Combination Therapy with GM-CSF. Clin. Cancer Res. 5(6) 1999: 1353–1361; https://pubmed.ncbi.nlm.nih.gov/10389919.
19 Bierich JR. Treatment of Pituitary Dwarfism with Biosynthetic Growth Hormone. Acta Paediatr. Scand. 75(s325) 1986: 13s–18s. https://doi.org/10.1111/j.1651-2227.1986.tb10357.x.
35 Hajam IA, et al. Bacterial Flagellin — A Potent Immunomodulatory Agent. Exper. Molec. Med. 49(9) 2017: e373; https://doi.org/10.1038/emm.2017.172.
36 Tenaillon O, et al. The Population Genetics of Commensal Escherichia coli. Nat. Rev. Microbiol. 8(3) 2010: 207–217; https://doi.org/10.1038/nrmicro2298.
37 Active and Passive Immunization. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Kimberlin D, et al., Eds. American Academy of Pediatrics: Itasca, IL, 2018: 13–64.
38 Pollard AJ, Bijker EM. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 21(2) 2021: 83–100; https://doi.org/10.1038/s41577-020-00479-7.
39 HogenEsch H, O’Hagan DT, Fox CB. Optimizing the Utilization of Aluminum Adjuvants in Vaccines: You Might Just Get What You Want. npj Vaccines 3, 2018: 51; https://doi.org/10.1038/s41541-018-0089-x.
40 Martiñón S, et al. Chemical and Immunological Characteristics of Aluminum-Based, Oil-Water Emulsion, and Bacterial-Origin Adjuvants. J. Immunol. Res. 8 May 2019: 3974127; https://doi.org/10.1155/2019/3974127.
41 Crépeaux G, et al. Low Concentrations of Aluminum Hydroxide Adjuvant, Forming Limited Size Aggregates, Selectively Induce Cerebral Aluminum Increase and Long-Term Neurotoxicity in Mouse. Morphologie 100(330) 2016: 160–161; https://doi.org/10.1016/j.morpho.2016.07.003.
42 Israeli E, et al. Adjuvants and Autoimmunity. Lupus 18(13) 2009: 1217–1225; https://doi.org/10.1177/0961203309345724.
43 Gherardi RK, et al. Macrophagic Myofasciitis Lesions Assess Long-Term Persistence of Vaccine-Derived Aluminum Hydroxide in Muscle. Brain 124(9) 2001: 1821–1831; https://doi.org/10.1093/brain/124.9.1821.
44 Chkheidze R et al. Morin Stain Detects Aluminum-Containing Macrophages in Macrophagic Myofasciitis and Vaccination Granuloma with High Sensitivity and Specificity. J. Neuropathol. Exper. Neurol. 76(4) 2017: 323–331; https://doi.org/10.1093/jnen/nlx011.
45 Ulanova M, et al. The Common Vaccine Adjuvant Aluminum Hydroxide Up-Regulates Accessory Properties of Human Monocytes via an Interleukin-4-Dependent Mechanism. Infect. Immun. 69(2) 2001:1151–1159; https://doi.org/10.1128/IAI.69.2.1151-1159.2001.
46 Strowig T, et al. Inflammasomes in Health and Disease. Nature 481(7381) 2012: 278–286; https://doi.org/10.1038/nature10759.
47 Wen H, Miao EA, Ting JP. Mechanisms of NOD-Like Receptor-Associated Inflammasome Activation. Immunity 39(3) 2013: 432–441; https://doi.org/10.1016/j.immuni.2013.08.037.
48 Yang X, et al. Autoproteolytic Activation of Procaspases By Oligomerization. Molec. Cell 1(2) 1998: 319–325; https://doi.org/10.1016/s1097-2765(00)80032-5.
49 Fantuzzi G, Dinarello CA. Interleukin-18 and Interleukin-1 Beta: Two Cytokine Substrates for ICE (Caspase-1). J. Clin. Immunol. 19(1) 1999: 1–11; https://doi.org/10.1023/a:1020506300324.
50 Stone KD, Prussin C, Metcalfe DD. IgE, Mast Cells, Basophils, and Eosinophils. J. Allergy Clin. Immunol. 125(suppl 2) 2010: s73–s80; https://doi.org/10.1016/j.jaci.2009.11.017.
51 Kalinski P, et al. T-Cell Priming by Type-1 and Type-2 Polarized Dendritic Cells: The Concept of a Third Signal. Immunol. Today 20(12) 1999: 561–567; https://doi.org/10.1016/s0167-5699(99)01547-9.
52 De Jong EC, Smits HH, Kapsenberg ML. Dendritic Cell-Mediated T-Cell Polarization. Springer Semin. Immunopathol. 26(3) 2005: 289–307; https://doi.org/10.1007/s00281-004-0167-1.
53 Ochsenbein AF, et al. Protective Long-Term Antibody Memory by Antigen-Driven and T Helper-Dependent Differentiation of Long-Lived Memory B cells to Short-Lived Plasma Cells Independent of Secondary Lymphoid Organs. Proc. Nat. Acad. Sci. USA, 97(24) 2000: 13263–13268; https://doi.org/10.1073/pnas.230417497.
54 Kumar H, Kawai T, Akira S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 30(1) 2011: 16–34; https://doi.org/10.3109/08830185.2010.529976.
55 Barton GM. Viral Recognition by Toll-Like Receptors. Semin. Immunol. 19(1) 2007: 33–40; https://doi.org/10.1016/j.smim.2007.01.003.
56 Xagorari A, Chlichlia K. Toll-Like Receptors and Viruses: Induction of Innate Antiviral Immune Responses. Open Microbiol. J. 2, 2008: 49–59; https://doi.org/10.2174/1874285800802010049.
57 Pandey S, Kawai T, Akira S. Microbial Sensing By Toll-Like Receptors and Intracellular Nucleic Acid Sensors. Cold Spring Harbor Perspect. Biol. 7(1) 2014: a016246; https://doi.org/10.1101/cshperspect.a016246.
58 Gu Y, et al. Activation of Interferon-Gamma Inducing Factor Mediated by Interleukin-1-Beta Converting Enzyme. Science 275(5297) 1997: 206–209; https://doi.org/10.1126/science.275.5297.206.
59 Sokol CL, Luster AD. The Chemokine System in Innate Immunity. Cold Spring Harbor Perspect. Biol. 7(5) 2015: a016303; https://doi.org/10.1101/cshperspect.a016303.
60 Hatai H, et al. Toll-Like Receptor 11 (TLR11) Interacts with Flagellin and Profilin Through Disparate Mechanisms. PLOS one 11(2) 2016: e0148987; https://doi.org/10.1371/journal.pone.0148987.
61 Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 33(4) 2010: 492–503; https://doi.org/10.1016/j.immuni.2010.10.002.
62 The Immunological Basis for Immunization Series: Module 4 — Pertussis. World Health Organization: Geneva, Switzerland, 2017; https://apps.who.int/iris/bitstream/handle/10665/259388/9789241513173-eng.pdf;jsessionid=D1FD2EE043E4FA918D8B1C979B482F7F?sequence=1.
63 Fluvacsin SV Ficha Técnica. Comisión Federal Para la Protección Contra Riesgos Sanitarios: Mexico City, Mexico, 4 November 2019; https://www.gob.mx/cms/uploads/attachment/file/173474/051M2013.pdf.
64 Pulmovax Ficha Técnica. Comisión Federal Para la Protección Contra Riesgos Sanitarios: Mexico City, Mexico, 4 November 2019; https://www.gob.mx/cms/uploads/attachment/file/192194/90825.pdf.
65 Hexacima Ficha Técnica. Comisión Federal Para la Protección Contra Riesgos Sanitarios: Mexico City, Mexico, 4 November 2019; https://www.gob.mx/cms/uploads/attachment/file/207524/063M2013.pdf.
66 Engerix Ficha Técnica. Comisión Federal Para la Protección Contra Riesgos Sanitarios: Mexico City, Mexico, 4 November 2019; https://www.gob.mx/cms/uploads/attachment/file/173519/119M87.pdf.
67 Shen H, et al. Neonatal Vaccination with Bacillus Calmette–Guérin Elicits Long-Term Protection in Mouse-Allergic Responses. Allergy 63, 2008: 555–563; https://doi.org/10.1111/j.1398-9995.2008.01637.x.
68 Fox B, et al. Efficacy of a Therapeutic Cocaine Vaccine in Rodent Models. Nature Med. 2(10) 1996: 1129–1132; https://doi.org/10.1038/nm1096-1129.
69 Hamouda T, et al. Intranasal Immunization of Ferrets with Commercial Trivalent Influenza Vaccines Formulated in a Nanoemulsion-Based Adjuvant. Clin. Vacc. Immunol. 18(7) 2011: 1167–1175; https://doi.org/10.1128/CVI.00035-11.
70 Melamed D, Friedman A. Direct Evidence for Anergy in T Lymphocytes Tolerized by Oral Administration of Ovalbumin. Eur. J. Immunol. 23(4) 1993:
935–942; https://doi.org/10.1002/eji.1830230426.
71 Dobrzynski E, et al. Induction of Antigen-Specific CD4+ T-Cell Anergy and Deletion by in Vivo Viral Gene Transfer. Blood 104(4) 2004: 969–977; https://doi.org/10.1182/blood-2004-03-0847.
72 Endres RO, Grey HM. Antigen Recognition by T Cells. II. Intravenous Administration of Native or Denatured Ovalbumin Results in Tolerance to Both Forms of the Antigen. J. Immunol. 125(4) 1980: 1521–1525; https://www.jimmunol.org/content/125/4/1521.
73 Friedman A, Weiner HL. Induction of Anergy or Active Suppression Following Oral Tolerance Is Determined by Antigen Dosage. Proc. Nat. Acad. Sci. USA 91(14) 1994: 6688–6692; https://doi.org/10.1073/pnas.91.14.6688.
74 Wang W, Singh M. Selection of Adjuvants for Enhanced Vaccine Potency. World J. Vaccines 1(2) 2011: 33–78; https://doi.org/10.4236/wjv.2011.12007.
75 Gelzleichter TR. Early Characterization of Biosimilar Therapeutics. Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics. Plitnick L, Herzyk D, Eds. Academic Press: Cambridge, MA, 2013: 185–210.
76 Huang LY, et al. Use of Toll-Like Receptor Assays to Detect and Identify Microbial Contaminants in Biological Products. J. Clin. Microbiol. 47(11) 2009: 3427–3434; https://doi.org/10.1128/JCM.00373-09.
77 Janeway CA Jr., et al. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Science: New York, NY, 2001.
78 Li B. Construction and Characterization of a Humanized Anti-Human CD3 Monoclonal Antibody 12F6 with Effective Immunoregulation Functions. Immunol. 116(4) 2005: 487–498; https://doi.org/10.1111/j.1365-2567.2005.02247.x.
79 Jaffers GJ, et al. Monoclonal Antibody Therapy. Anti-Idiotypic and Non-Anti-Idiotypic Antibodies to OKT3 Arising Despite Intense Immunosuppression. Transplantation 41(5) 1986: 572–578; https://doi.org/10.1097/00007890-198605000-00004.
80 Pascual-Salcedo D, et al. Influence of Immunogenicity on the Efficacy of Long-Term Treatment with Infliximab in Rheumatoid Arthritis. Rheumatology (UK) 50(8) 2011: 1445–1452; https://doi.org/10.1093/rheumatology/ker124.
81 Garcês S, Demengeot J, Benito-Garcia E. The Immunogenicity of Anti-TNF Therapy in Immune-Mediated Inflammatory Diseases: A Systematic Review of the Literature with a Meta-Analysis. Ann. Rheum. Dis. 72(12) 2013: 1947–1955; https://doi.org/10.1136/annrheumdis-2012-202220.
82 de Vries MK, et al. Immunogenicity Does Not Influence Treatment with Etanercept in Patients with Ankylosing Spondylitis. Ann. Rheum. Dis. 68(4) 2009: 531–535; https://doi.org/10.1136/ard.2008.089979.
83 Harding FA, et al. The Immunogenicity of Humanized and Fully Human Antibodies: Residual Immunogenicity Resides in the CDR Regions. mAbs 2(3) 2010: 256–265; https://doi.org/10.4161/mabs.2.3.11641.
84 Jones AJ. The Use of an Animal Immunogenicity Model in the Development of Protropin Somatrem (Methionyl Human Growth Hormone). Dev. Biol. (Basel) 109, 2002: 107–118.
85 Poolman JT. Shortcomings of Pertussis Vaccines: Why We Need a Third-Generation Vaccine. Exp. Rev. Vaccines 13(10) 2014: 1159–1162; https://doi.org/10.1586/14760584.2014.944902.
86 Li-Kim-Moy J, Booy R. The Manufacturing Process Should Remain the Focus for Severe Febrile Reactions in Children Administered an Australian Inactivated Influenza Vaccine During 2010. Influenza and Other Respiratory Viruses 10(1) 2016: 9–13; https://doi.org/10.1111/irv.12337.
87 Epogen (Recombinant Epoetin Alfa): Resources. Amgen, accessed 27 August 2022; https://www.epogen.com/resources.
88 Rubio C, et al. Anafilaxia. Anales Sis. San. Navarra 26(suppl 2) 2003: 103–110; https://scielo.isciii.es/scielo.php?pid=S1137-66272003000400013&script=sci_abstract&tlng=en.
89 Weber G, et al. Allergic Skin and Systemic Reactions in a Patient with Pure Red Cell Aplasia and Anti-Erythropoietin Antibodies Challenged with Different Epoetins. J. Amer. Soc. Nephrol. 13(9) 2002: 2381–2383; https://doi.org/10.1097/01.ASN.0000027031.79843.6C.
90 García J, et al. Anaphylactic Reaction to Human Recombinant Erythropoietin: Renal Unit and Allergy Unit, Virgen de la Salud Hospital. Nephron 65, 1993: 636–637; https://www.karger.com/Article/Pdf/187578.
91 Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: An Investigation into Its Epidemiology. Arch. Intern. Med. 161(1) 2001: 15–21; https://doi.org/10.1001/archinte.161.1.15.
92 Pumphrey RS. Fatal Anaphylaxis in the UK, 1992–2001. Proc. Novartis Found. Symp. 257, 2004: 116–132, 157–160, 176–185.
93 Ampon RD, et al. Impact of Asthma on Self-Reported Health Status and Quality of life: A Population-Based Study of Australians Aged 18–64. Thorax 60(9) 2005: 735–739; https://doi.org/10.1136/thx.2005.040311.
94 Liew W., Williamson E, Tang ML. Anaphylaxis Fatalities and Admissions in Australia. J. Allergy Clin. Immunol. 123(2) 2009: 434–442; https://doi.org/10.1016/j.jaci.2008.10.049.
95 Pumphrey R. Anaphylaxis: Can We Tell Who Is at Risk of a Fatal Reaction? Curr. Opin. Allergy Clin. Immunol. 4(4) 2004: 285–290; https://doi.org/10.1097/01.all.0000136762.89313.0b.
96 Poulos LM, et al. Trends in Hospitalizations for Anaphylaxis, Angioedema, and Urticaria in Australia, 1993–1994 to 2004–2005. J. Allergy Clin. Immunol. 120(4) 2007: 878–884; https://doi.org/10.1016/j.jaci.2007.07.040.
97 Kamijo S, et al. Subcutaneous Allergic Sensitization to Protease Allergen Is Dependent on Mast Cells but Not IL-33: Distinct Mechanisms Between Subcutaneous and Intranasal Routes. J. Immunol. 196(9) 2016: 3559–3569; https://doi.org/10.4049/jimmunol.1500717.
98 Milne J, Brand S. Occupational Asthma After Inhalation of Dust of the Proteolytic Enzyme, Papain. Br. J. Ind. Med. 32(4) 1975: 302–307; https://doi.org/10.1136/oem.32.
4.302.
99 Gailhofer G, et al. Asthma Caused by Bromelain: An Occupational Allergy. Clin. Exp. Allergy 18(5) 1988: 445–450; https://doi.org/10.1111/j.1365-2222.1988.tb02894.x.
100 Simons FER. Anaphylaxis. J. Allergy Clin. Immunol. 125(2) 2010: s161–s181; https://doi.org/10.1016/j.jaci.2009.12.981.
101 Shimokawa C, et al. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity 46(5) 2017: 863–874; https://doi.org/10.1016/j.immuni.2017.04.017.
102 Bjornsson HM, Graffeo CS. Improving Diagnostic Accuracy of Anaphylaxis in the Acute Care Setting. West. J. Emerg. Med. 11(5) 2010: 456–461; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3027438.
103 Ring J, et al. Guideline for Acute Therapy and Management of Anaphylaxis. Allergo J. Int. 23(3) 2014: 96–112; https://doi.org/10.1007/s40629-014-0009-1.
104 Lee JK, Vadas P. Anaphylaxis: Mechanisms and Management. Clin. Exper. Allergy 41, 2011: 923–938; https://doi.org/10.1111/j.1365-2222.2011.03779.x.
105 FDA Warns About Rare but Serious Allergic Reactions with the Skin Antiseptic Chlorhexidine Gluconate. US Food and Drug Administration: Silver Spring, MD,
2 February 2017; https://www.fda.gov/media/102986/download.
106 Serious Allergic Reactions Reported with the Use of Saphris (Asenapine Maleate). US Food and Drug Administration: Silver Spring, MD, 1 September 2011; https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-serious-allergic-reactions-reported-use-saphris-asenapine-maleate.
107 Full Prescribing Information: FIASP (Insulin Aspart Injection) for Subcutaneous or Intravenous Use. US Food and Drug Administration: Silver Spring, MD, December 2019 (revised); https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208751s010s011lbl.pdf.
108 Vultaggio A, et al. Anti-Infliximab IgE and Non-IgE Antibodies and Induction of Infusion Related Severe Anaphylactic Reactions. Allergy 65, 2010: 657–661; https://doi.org/10.1111/j.1398-9995.2009.02280.x.
109 Baert F, et al. Influence of Immunogenicity on The Long-Term Efficacy of Infliximab in Crohn’s Disease. New Engl. J. Med. 348(7) 2003: 601–608; https://doi.org/10.1056/NEJMoa020888.
110 Xue L, Johnson R, Gorovits B. Prevalence and Isotypic Complexity of the Anti-Chinese Hamster Ovary Host Cell Protein Antibodies in Normal Human Serum. AAPS J. 12(1) 2010: 98–106; https://doi.org/10.1208/s12248-009-9165-5.
111 Ingerslev J, et al. Antibodies to Heterologous Proteins in Hemophilia A Patients Receiving Recombinant Factor VIII (Recombinant). J. Thrombosis Haemostasis 87(4) 2002: 626–634; https://doi.org/10.1055/S-0037-1613059.
112 Singh SK, et al. Understanding the Mechanism of Copurification of “Difficult to Remove” Host Cell Proteins in Rituximab Biosimilar Products. Biotechnol. Prog. 36(2) 2020: e2936; https://doi.org/10.1002/btpr.2936.
113 Koren E, Zuckerman LA, Mire-Sluis AR. Immune Responses to Therapeutic Proteins in Humans: Clinical Significance, Assessment, and Prediction. Curr. Pharm. Biotechnol. 3(4) 2002: 349–360; https://doi.org/10.2174/1389201023378175.
114 Barger TE, et al. Detection of Anti-ESA Antibodies in Human Samples From PRCA and Non-PRCA Patients: An Immunoassay Platform Comparison. Nephrol. Dialysis Transplant. 27(2) 2012: 688–693; https://doi.org/10.1093/ndt/gfr213.
115 Dessypris EN. Pure Red Cell Aplasia. Hematology: Basic Principles and Practice. 3rd ed. Hoffman R, et al., Eds. Churchill Livingstone: New York, NY, 1999: 342–354.
116 McKoy JM, et al. Epoetin-Associated Pure Red Cell Aplasia: Past, Present, And Future Considerations. Transfusion 48(8) 2008: 1754–1762; https://doi.org/10.1111/j.1537-2995.2008.01749.x.
117 Locatelli F, Del Vecchio L, Pozzoni P. Pure Red-Cell Aplasia “Epidemic” — Mystery Completely Revealed? Perit. Dial. Int. 27(suppl 2) 2007: s303–s307.
118 Casadevall N, et al. Pure Red-Cell Aplasia and Antierythropoietin Antibodies in Patients Treated with Recombinant Erythropoietin. New Engl. J. Med. 346(7) 2002: 469-475; https://doi.org/10.1056/nejmoa011931.
119 Boven K, et al. The Increased Incidence of Pure Red Cell Aplasia with an Eprex Formulation in Uncoated Rubber Stopper Syringes. Kidney Int. 67(6) 2005: 2346–2353; https://doi.org/10.1111/j.1523-1755.2005.00340.x.
120 Rahbar M, et al. Pure Red Cell Aplasia Due to Antibody Against Erythropoietin in Hemodialysis Patients. J. Nephropathol. 6(1) 2017: 25–29; https://doi.org/10.15171/jnp.2017.05.
121 Palm NW, Medzhitov R. Immunostimulatory Activity of Haptenated Proteins. Proc. Nat. Acad. Sci. USA 106(12) 2009: 4782–4787; https://doi.org/10.1073/pnas.0809403105.
122 Birch K, Pearson-Shaver AL. Allergy Testing. StatPearls. StatPearls Publishing: Treasure Island, FL, 2021; https://www.ncbi.nlm.nih.gov/books/NBK537020.
123 Mehlhop-Williams ER, Bevan MJ. Memory CD8+ T Cells Exhibit Increased Antigen Threshold Requirements for Recall Proliferation. J. Exp. Med. 211(2) 2014: 345–356; https://doi.org/10.1084/jem.20131271.
124 Beatson R, et al. Transforming Growth Factor-Β1 Is Constitutively Secreted By Chinese Hamster Ovary Cells and Is Functional in human cells. Biotechnol. Bioeng. 108(11) 2011: 2759–2764; https://doi.org/10.1002/bit.23217.
125 Abe MJG, et al. An Assay for Transforming Growth Factor-β Using Cells Transfected with a Plasminogen Activator Inhibitor-1 Promoter-Luciferase Construct. Anal. Biochem. 216(2) 1994: 276–284; https://doi.org/10.1006/abio.1994.1042.
126 Reinhold D, et al. Increased Blood Plasma Concentrations of TGF-β Isoforms After Treatment with Intravenous Immunoglobulins (IVIG) in Patients with Multiple Sclerosis. J. Neuroimmunol. 152(1) 2004: 191–194; https://doi.org/10.1016/j.jneuroim.2004.03.018.
127 Annes JP, Munger JS, Rifkin DB. Making Sense of Latent TGFb Activation. J. Cell Sci. 15(116) 2003: 217–224; https://doi.org/10.1242/jcs.00229.
128 Jayapal KP, et al. Recombinant Protein Therapeutics from CHO Cells — 20 Years and
Counting. Chem. Eng. Prog. 103(10) 2007: 40–47; https://www.aiche.org/sites/default/files/docs/pages/CHO.pdf.
129 Haggerty H. Identification of Hamster MCP-1 Host Cell Protein Impurity as the Potential Culprit for Clinical Adverse Events (presentation). Charles River Biotechnology Symposium: San Diego, CA, 8–10 September 2014.
Víctor Pérez Medina Martínez and Carlos Eduardo Espinosa-de la Garza work in the research and development unit, and corresponding author Néstor O. Pérez works in operations management at Probiomed S.A. de C.V., Cruce de Carreteras Acatzingo-Zumpahuacán s/n, Tenancingo, Estado de México, México. C.P. 52400; 52-55-1166-2305; [email protected]; https://www.probiomed.com.mx.
You May Also Like