Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
In many ways, cells are the ultimate therapeutic product. They can integrate and participate in different biologic processes and replace missing biological functions. Cell therapies are dynamic, versatile, and with the appropriate engineering, capable of influencing and correcting disease processes robustly. Cell therapies essentially are living medicines, and their adaptability contrasts with conventional drug modalities that generally have only a single specific target or effect.
Because cell therapies are highly complex modalities, their scientific and R&D challenges are different from those of other biopharmaceuticals. No “template” approach for cell therapy production exists, and biomanufacturers cannot adapt technologies directly from small-molecule or antibody manufacturing workflows. A cell therapy manufacturing process is connected integrally to early development steps such as product design and to downstream operations such as formulation and cryopreservation.
New technologies for streamlining processing and manufacturing of cell therapies are essential to realizing their full potential, particularly their use as allogeneic (off-the-shelf) drug products. Innovation is needed to achieve consistent and controlled cell production for chimeric antigen receptor (CAR) T cells, CAR natural killer (NK) cells, stem cells, and other cell types. Robust methods for cell characterization and product testing must be developed and applied to ensure safe and robust manufacturing processes that follow good manufacturing practice (GMP) principles.
Astellas is making long-term investments in cell-therapy scientific research with the hopes of major therapeutic breakthroughs for patients. The company’s strategic initiatives have brought together capabilities that represent the Astellas Institute for Regenerative Medicine (AIRM). Located in the Boston biotechnology region, it is one of the most comprehensive regenerative medicine centers in the biopharmaceutical industry.
AIRM’s goal is to advance regenerative medicines to be off-the-shelf cell therapies that are designed to address multiple diseases and bring value to patients with unmet needs. The institute is establishing capabilities spanning pluripotent cell development, cell differentiation process development, and manufacturing. It is taking important steps toward redefining cell therapies for treating a vast number of diseases. AIRM’s researchers are investigating pluripotent stem cells (PSCs) as the starting material for allogeneic cell therapies that can rejuvenate, regenerate, and replace damaged tissues.
To achieve its vision of reaching many diseases and patients, AIRM is focused on applying technology advances in several areas that are central to the cell therapy value chain, including cell engineering, gene editing, automation, and cryopreservation.
Universal Donor Cell Technology
Many of the cell therapy programs at Astellas start from a PSC source. This allogeneic approach leverages renewable starting material to enable a consistent and replenishable cell therapy product for a large number of patients. Establishing a platform based on a nearly inexhaustible supply of PSCs is essential for implementing large-scale, off-the shelf production. That can’t be achieved for autologous therapies or therapies based on donor-dependent cell sources. Constraints related to processing autologous therapies include the limited number of locations and centers that can perform leukapheresis and cell processing and the short timelines required for product manufacturing and release testing. Likewise, tissue-derived sources are constrained by limited expandability of starting materials and donor-to-donor variability in quality and attributes that are essential for creating off-the-shelf cell therapies. Astellas takes a PSC-based allogeneic approach, enabled by universal donor-cell technology, to overcome those challenges and democratize access to cell therapies.
Universal donor-cell technology is the key to developing cell therapy products for all human leukocyte antigen (HLA) haplotypes. In conventional transplantation for allogeneic recipients, multiple HLA class I and class II proteins must be matched for histocompatibility. PSC-derived cells that have not been engineered to prevent allogeneic rejection are eliminated rapidly after transplantation unless tailored steps are taken for HLA-matching the cells to their recipients, delivering the cell therapies to immune-privileged sites such as the eyes or central nervous system, or subjecting patients to potentially dangerous immunosuppression.
In 2018, Astellas acquired Universal Cells, which has a unique technology for lowering immunological rejection potential of PSCs as a cornerstone capability for creating allogeneic therapies (Figure 1). Because of that reduced risk of rejection, the strategy might enable transition of different cell therapies based on a universal donor cell foundation. Astellas also uses a recombinant adenoassociated virus (AAV)–mediated gene-editing technology, which offers several advantages over an approach based on clustered regularly interspaced short palindromic repeats (CRISPR). Such advantages include the elimination of double-stranded DNA breaks, which is designed to optimize the editing process with reduced incidence of off-target editing errors.
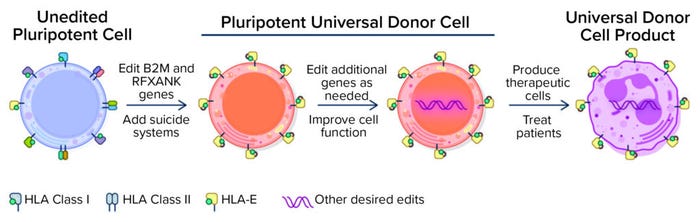
Figure 1: Universal donor cells are appropriate for developing numerous differentiated cell therapy products. (HLA = human leukocyte antigen).
Universal Cells’s gene-editing technology focuses on reducing the possibility of rejection by manipulating HLA expression in stem cells. Gene edits knock out the expression of HLA class I and class II surface proteins that are thought to be largely responsible for eliciting T-cell responses and immune rejection. Another gene edit leads to expression of specific nonpolymorphic HLA molecules to provide essential class I signals that block lysis by NK cells. A suicide gene, such as thymidine kinase (TK), can be engineered into cells for enhanced safety. Upon exposure to ganciclovir, TK catalyzes the generation of toxic by-products, thus killing off cells that express the TK suicide gene. Although stringent quality control and release criteria for cell therapy products are likely to obviate the need to use a suicide gene, its presence provides an important safety net. With that editing technology, Astellas also uses a sophisticated bioinformatics platform to screen cells and verify that they have been edited and are genetically stable.
Engineered CAR Cell Therapies
In 2019, Astellas acquired Xyphos Biosciences and its proprietary CAR technology for using synthetic biology methods to adapt cell therapies with improved flexibility, increased control, and enhanced safety. The convertibleCAR technology platform enables production of cell therapies that can target more than one tumor antigen on cancer cells, thus offering improved ways to mobilize immune cells to find and destroy target tumor cells. The convertibleCAR cells produced through the platform can be directed specifically to tumor cells, and with activity toward tumor destruction tightly controlled from outside a CAR cell, thereby leading to safe and efficacious cancer treatments.
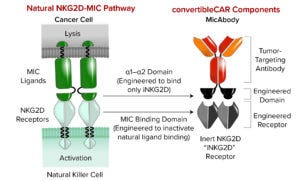
Figure 2: Astellas’s convertibleCAR technology creates flexible cell therapies that can be engineered and reengineered inside the body. (MIC = major histocompatibility complex class I chain-related, NKG = natural killer group).
The convertibleCAR technology uses a modified ligand-binding domain from the natural human immune-activating receptor NKG2D as the externally facing portion of a CAR (Figure 2). That enables activity of the CAR to be controlled through precise binding of a bispecific molecule — a MicAbody antibody — to the modified NKG2D on one end and a tumor-associated antigen on the other end. That approach can enable specific targeting of tumor cells by bringing them close to CAR-expressing immune cells, which can then be activated to attack and destroy tumor cells.
By attaching different functional molecules, the convertibleCAR therapy can be adapted to target different tumor antigens, even as tumors change and evolve to evade detection by the immune system. Thus, this technology potentially overcomes the challenges of tumor resistance. Multiple antigens also can be targeted by using different MicAbody antibodies, ensuring that the cells aren’t overly active. The cells can be controlled in terms of when they are “on” or “off” because a MicAbody antibody functions as an “on” switch. Traditional CARs always are active. That leads to concerns about cytokine release syndrome and heightened activity of cells. The convertibleCAR technology enables a CAR construct to be inert until a MicAbody antibody is introduced, thus enabling control over when a receptor is active, with response titration and versatile targeting. Astellas plans to use the convertibleCAR technology in a foundational PSC to create one master cell bank. A library of MicAbody antibodies would serve as adapters for specific targets. That approach could reduce the need to reengineer cells for different targets, as is necessary for conventional CAR cells.
Automation and Cryopreservation
The scale of manufacturing an off-the-shelf allogeneic cell therapy product is exponentially larger than that needed for a patient-specific autologous therapy. An allogeneic therapy can treat hundreds or thousands of patients, so the ability to scale up cell production efficiently and cost effectively is crucially important. Automation is invaluable because successful scale-up relies on the ability to control a wide range of parameters. Automation can be applied to a cryopreservation process when aliquoting precise numbers of cells into individual vials and to the freezing process itself. Cells must be frozen gradually to a cryopreservable temperature without destroying or disrupting them. Automated technologies can facilitate and standardize that process.
With central manufacturing of allogeneic cell therapies, rapid and secure cold-chain shipping to patients around the world also is important. A cell therapy must remain at a specific temperature throughout transit and upon arrival at the intended destination. The product must be handled and stored properly until needed.
Astellas also focuses on the development of formulation buffers that preserve cell viability and support rapid and easy thawing of a cryopreserved cell therapy once the product arrives at the delivery site. An optimized buffer is used to thaw vials and dilute their contents so that the cells can be injected. That process does not require someone to thaw the vials, spin down the cells, remove the cryopreservative, and reconstitute the cells in another buffer — activities that can damage cells, especially those that are fragile or have difficulty withstanding a freeze–thaw process.
A Promising Future
Cell therapies are some of the most promising new drug modalities, representing a significant step forward in treating a broad range of diseases and conditions. Stem-cell–based therapy candidates already are in clinical trials for regenerative medicines that can be administered directly to a patient’s eye for treatment of ocular diseases. Astellas’s work with allogeneic cells is driving toward regenerative medicines in several therapeutic areas, including immunology and cardiovascular disease. The company is starting with autologous, cancer-focused CAR-T therapies and moving into scalable, allogeneic CAR-NK therapies to treat multiple types of tumors. Those engineered cell therapies require a suite of advanced technologies to design and manufacture them with properties of safety, tumor targeting, and cytotoxic activity. Astellas is working to overcome challenges in its processing and production to realize the goal of making those products at large scale for off-the-shelf use. The goal is to create new allogeneic cell therapy solutions by bringing together leading-edge technologies such as those described herein.
Erin Kimbrel, PhD, is vice president of research at the Astellas Institute for Regenerative Medicine (AIRM); [email protected]. Gary Starling, PhD, is chief scientific officer at Xyphos and Center of Excellence for Cancer Cell Therapy; [email protected].
You May Also Like