Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
January 31, 2020
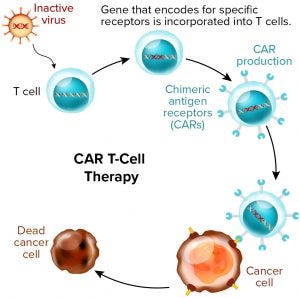
Figure 1: Chimeric antigen receptor (CAR) T-cell therapies also are gene therapies. (www.istockphoto.com)
Before we had the 21 CFR 1271 regulation for tissue therapies, the US Food and Drug Administration (FDA) had determined that regenerative medicine was exceptional enough to warrant its own regulations for good manufacturing practice (GMP). Since 2001, the tissue industry has adapted to those new rules while the FDA stepped up enforcement over time. When a cell or tissue product is regulated under 21 CFR 1271, its specific regulations apply before the general regulations for biologics and drugs. But what about combined gene and cell therapies? Are they covered under existing regulations and guidances, or does this new product class warrant its own GMPs within the existing framework for drugs, biologics, and tissue products?
Talk has circulated recently about the possible need for separate regulations for cell and gene therapies. But I do not think that we need separate GMPs for this product class. Gene therapies often involve viral vectors such as adenoassociated viruses (AAVs) or lentiviruses, and they can involve DNA or RNA sequences and — as in the case of chimeric-antigen receptor (CAR) T cells — human cells that are genetically modified ex vivo.
Viral vectors usually are expressed by eukaryotic cells through standard cell culture bioprocesses in bioreactors. The cells are subjected to a modified virus that replicates in them for subsequent harvest as the active pharmaceutical ingredient (API) or bulk drug substance (DS). Such a production process is very similar to that of cell-based vaccine manufacturing, in which antigens or virus-like particles are made using similar cell cultures. Regulators have experience with such products and treat them as biologics.
Although that is only one approach, I argue that most if not all gene therapy processes are similar to such biotechnology processes as cell-based antigen production. For example, ex-vivo modified human cells are similar to other types of cell therapies (in a GMP sense) with the addition of genetic manipulation. Similar concerns apply for all gene therapy products from a GMP perspective: e.g., requiring good assays for safety, quality, identity, potency, and purity, along with reliable in-process controls and controls for aseptic processing.
The FDA groups cell and gene therapies together as “advanced therapies” regulated by the Center for Biologics Evaluation and Research (CBER) and the Office of Tissues and Advanced Therapies (OTAT). A recent draft guidance (1) notes that gene therapy products meet the definition of “biological products” and thus are covered by both the drug GMPs (21 CFR 210 and 211) and biologics regulations (21 CFR 600s). This important document represents the agency’s current thinking about what should be contained within the chemistry, manufacturing, and controls (CMC) section of cell/gene-therapy investigational new drug (IND) applications. Thus, it would also apply to the CMC section of their biologics license applications (BLAs) — which would be more extensive, of course. So the FDA has communicated its thoughts on what is required for gene therapy applications in addition to current regulation. The agency also might write additional gene therapy product guidances as it deems necessary to outline its current thinking on licensure (BLA) requirements further.
Because gene-therapy products are classified as biologics — and therefore are drugs under the law — they already are covered under current drug and biologics regulations. In addition, other specific guidances may be of interest (as detailed below). Because some gene therapies combine cell therapy, cell culture, and gene sequences, the cell therapy guidances would be of interest and applicable in such cases. CAR T cells are a prime example.
The FDA already has some precedent for regulating AAV vectors and CAR-T therapies: Some such products have been approved already. Several gene therapy products are now on the market in the United States, thus validating the agency’s commitment to regulating and approving such advanced therapies without separate GMP regulations. The regulatory pathway has been defined — and with this precedent, more therapies will be approved.
European gene therapy guidance is in place as well and provides a comprehensive overview of GMP manufacturing for gene therapy products (2). Most of the information is standard for biologics, with some specific information added for gene therapies. Examples include the statement for physical separation of vectors within a manufacturing facility: Section 4.16 states, “Special precautions should be taken in the case of manufacturing activities involving infectious viral vectors (e.g., oncolytic viruses): these activities should take place in a segregated area” (2). The document emphasizes conducting risk assessments, particularly for evaluating concurrent production of two different batches or evaluating multiproduct manufacturing, to prevent risks and mix-ups.
In addition to the standard GMPs, the European guidance is specific to principles applied to starting material banks for vectors: Section 7.36 states, “In the case of vectors and naked plasmids used as starting materials for the manufacturing of gene therapy medicinal products, the principles of GMP apply from the bank system used to manufacture the vector or plasmid used for gene transfer” (2). The guidance also recommends establishing master and working seed lots and cell banks under GMP compliance (section 8.11). Vectors require containment measures: biosafety level 2 (BSL2) and/or negative-pressure areas, as is mentioned in Section 15.11: “Containment measures should be established according to the risk of the product that is handled, including measures regarding the design of the premises, organizational and technical measures, and measures regarding the treatment of residues” (2). Aseptic processing also is noted as important for gene therapies.
Thus, several important guidance documents are available to help developers interpret the GMP regulations for gene therapy products. Below I highlight some CMC-relevant guidances from the FDA and summarize some points of the first CMC-specific guidance that are specifically relevant to gene therapies.
FDA Guidances Specific to CMCs
The FDA has published several draft and final gene therapy guidances with CMC information (1, 3–5). Clinical, preclinical, and non-CMC guidances are outside the purview of this discussion. I concentrate here on the CMC guidance, which for now is still in draft form (1). All information submitted with an IND also should appear in the subsequent biologics license application (BLA). Some elements of this guidance differ from the expectations for biologics in general. For example, the agency notes on page 7 that sponsors should submit information on molecular structure, including genetic sequence and/or cellular components. The preferred format is a schematic sequence diagram showing promoters/enhancers, introns, and other relevant signals; restriction sites; and functional components (transgenes and markers). That sequence data should be submitted in Section 3.2.S.3.1, along with information supporting genetic stability.
The guidance describes expectations for information submitted about viral vectors, including such elements as biophysical characteristics and the nature of the viral genome. Expectations for descriptive information about bacterial vectors and ex-vivo genetically modified cells (e.g., CAR T cells) also are included. For the latter, the guidance recommends including a description of expected cell populations and the vector used to transferred a transgene into those cells. For those modified using genome editing, the altered genes should be described along with how the change is made and what technology is used.
Biomanufacturing processes and their controls always must be described according to Section 3.2.S.2.2 of the common technical document (CTD) guidance (6). But the CMC guidance notes some additional salient points for gene therapy products (1): The DS process description should include (as applicable) cell culture, transduction, cell expansion, harvest, purification, drug-product filling, storage, and shipping. Also note that time limits and stability information for all hold steps be listed when a BLA is filed. The process description also should cover process controls. Batch scale should be defined, and how the DS is quantified (vector viral particles, vector genomes, mass, number of genetically modified cells, etc.) should be spelled out.
Cell culture and vector production are common steps in gene therapy production, and the guidance notes related information that should be included. Detailed descriptions of cell culture conditions and process steps should include whether they are open or closed, what monitoring is performed, the length of hold times, and how transfers are made. Vector-production processes should be elucidated with all production and purification processes/procedures detailed. Those normally would encompass such information as a host-cell description and delineation of all steps in cell culture and expansion, transfection/infection, harvest, purification, concentration, and/or buffer exchange.
If the ultimate product is genetically modified cells (e.g., CAR T cells), then a complete description of their processing would cover their source (allogeneic or autologous), collection procedures, storage, shipping/handling, cell selection, isolation or enrichment, cell expansion process and conditions, hold times, transfers, harvest, purification, and all associated materials. Labeling and information recorded for all those steps also are important details, especially for autologous cell therapies. Applicants should provide a full description of how gene modification is performed, including such steps as transfection, infection, vector electroporation, genome editing, additional expansion, cell selection, or other treatments that follow modification.
The guidance also covers cell/virus banking, noting that the history and derivation of source material should be described along with all testing to characterize the bank. When cell substrates are genetically modified, the materials used should be characterized sufficiently to ensure safety and purity of the final product. All banked materials need to be qualified to establish the safety, identity, purity, and stability of cells used. Virus-bank information should include a detailed description of the history and derivation of seed materials. The guidance notes that a gene map of the final vector (and intermediates if applicable) will be useful, as will a description of how seed material was purified. Information also is needed on how a virus bank is qualified for safety, identity, purity, and stability — along with storage conditions. It’s important to demonstrate the absence of replication-competent viruses in replication-incompetent vector pools along with other qualification tests, such as ensuring correct genetic sequences.
Gene Therapy Manufacturing Facilities
Facility considerations for gene therapy products are similar to those for all other biotechnology and cell therapy products. Some important differences relate to special controls for vectors. Vector production often is contained in a biosafety level two (BSL2) area with appropriate controls. Also common in manufacturing vectors is use of negative-pressure zones to isolate possible spills. Pressurizations ideally should be arranged to create a pressure cascade from cleaner to less so areas and use negative pressure to isolate possible vector noncontainment. Use of both closed and single-use systems is common in such settings, with both serving to isolate vectors from the surrounding environment.
Cell culture within closed and single-use systems also can be aseptic, therefore preventing contamination from the environment while also preventing vector leaks into the environment after cells have been infected. Because facility design can be quite complicated for vector production and gene therapies in general, qualified consultants should review the flows (of personnel, product, waste, and materials), pressurizations, classifications, cleanroom construction, equipment arrangement, space considerations, and so on.
Consideration should be given to use of vaporized hydrogen peroxide (VHP) for cleaning production areas, especially for multiproduct areas where viral vectors are produced. For example, a facility design ideally should include a provision for isolating rooms in which viral vector material spills can occur and for subsequent fumigation with VHP. That option is helpful both for multiproduct areas and general vector production. VHP normally would not be performed routinely for biomanufacturing using closed systems, although some manufacturers do use it for changeover in multiproduct areas. Single-pass air can be used within production rooms that are BSL2 or viral-vector production areas. It facilitates isolation for vector production — again, in case a spill occurs — and for multiproduct areas.
All cleanroom concerns related to biologics/biotechnology in general also apply to gene therapy facilities. Cleanrooms need smooth and cleanable surfaces, high-efficiency particulate air (HEPA) filtration, pressurization, adequate lighting, material and personnel airlocks (ideally with interlocks), qualified critical utilities and equipment, and so on.
The Current State of Affairs
In my auditing practice over the past decade or so, I have seen many GMP gaps in production of gene therapies. Most gaps were much the same as for biologic products in general, however: e.g., problems with validation, materials, testing, characterization, process validation, and labeling/packaging. Few were specific to gene therapies, and those that were often related to documentation: e.g., risk assessment, written rationale for a specific practices and acceptance criterion, and so on.
In one case, gowning was insufficient for viral-vector production, and staff members were wearing their scrubs in common areas, which is a poor practice when viral-vector materials could be on those gowning materials. In another case, heating, ventilation, and air conditioning (HVAC) controls were insufficient for a multiproduct area where different viral vectors were manufactured as drug substances. The HVAC system had to be changed so that pressurizations could be maintained while single-pass air and negatively pressurized airlocks were being used. Even in these more specific cases, you can imagine another biopharmaceutical product (not gene or cell therapy) made with similar GMP gaps.
I do not believe that new GMP regulations are needed for gene/cell therapy products. But that does not mean that guidance documents are unnecessary. They are very helpful to companies putting marketing applications together and to the regulators who review those applications. When I was at the FDA as a reviewer, we were required to follow the guidances in our reviews and inspections because those consensus documents are vetted by many industry experts and regulators. Several good gene and cell therapy guidances are available both from the FDA and the European Medicines Agency (EMA) to assist the industry in gearing up for and compiling regulatory applications. We have long lists of what should be included and thus a framework for both INDs and BLAs to support new cell/gene therapies coming through the product pipeline.
References
1 CBER. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs): Draft Guidance for Industry. US Food and Drug Administration: Rockville, MD, July 2018; www.fda.gov/media/113760/download.
2 EudraLex. Guidelines on Good Manufacturing Practice Specific to Advanced Therapy Medicinal Products. European Commission: Brussels, Belgium, November 2017; https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/2017_11_22_guidelines_gmp_for_atmps.pdf.
3 CBER. Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture and Patient Follow-Up. US Food and Drug Administration: Rockville, MD, July 2018; www.fda.gov/media/113790/download.
4 CBER. Recommendations for Microbial Vectors Used for Gene Therapy. US Food and Drug Administration: Rockville, MD, September 2016; www.fda.gov/media/94200/download.
5 CBER. Potency Tests for Cellular and Gene Therapy Products. US Food and Drug Administration: Rockville, MD, January 2011; www.fda.gov/media/79856/download.
6 CBER/CDER. Providing Regulatory Submissions in Electronic Format — Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications: Guidance for Industry. US Food and Drug Administration: Rockville, MD, January 2019; www.fda.gov/media/120094/download.
John R. Godshalk is senior consultant for GMP, CMC, and quality at Biologics Consulting, 1555 King Street, Suite 300, Alexandria, VA 22314; 1-301-221-8247; [email protected]; www.biologicsconsulting.com.
You May Also Like