Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
 Cell and gene therapy manufacturing is about to hit a breaking point. The tension lies between increasingly diverse research pipelines and a tradition of dedicated facilities built for single-product, large-scale manufacturing. That incompatibility is widening as more cell and gene therapy products progress toward commercial production, forcing manufacturers to make a choice: either invest in major facility modifications and complex technology transfers to keep up or break from tradition and explore the potential of multimodal manufacturing.
Cell and gene therapy manufacturing is about to hit a breaking point. The tension lies between increasingly diverse research pipelines and a tradition of dedicated facilities built for single-product, large-scale manufacturing. That incompatibility is widening as more cell and gene therapy products progress toward commercial production, forcing manufacturers to make a choice: either invest in major facility modifications and complex technology transfers to keep up or break from tradition and explore the potential of multimodal manufacturing.
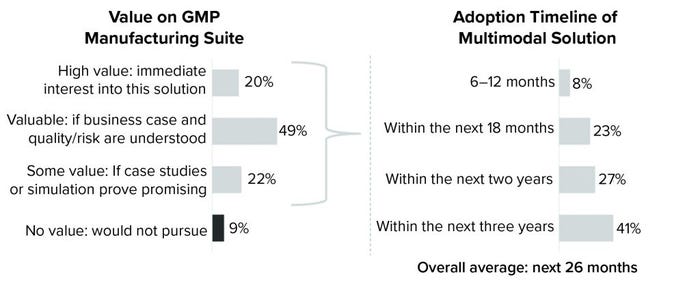
More than half of cell and gene therapy manufacturers plan to adopt multimodal solutions within the next two years, according to the Horizons: Cell and Gene Therapy industry survey conducted by CRB last year (Figure 1). With viral vectors, autologous cell therapies, and monoclonal antibodies (MAbs) currently dominating research pipelines, those manufacturers say that flexibility, scalability, and speed to market are driving them away from dedicated facilities. The key is being ready to move — and to move fast — without compromising product integrity and patient safety. To get there, however, the cell and gene industry must realize a seismic shift in the way that it thinks about manufacturing.
Preparing for a Multimodal Mindset
Like previous manufacturing shifts in the bioprocess industry, this one will come in waves. Think back to the early days of MAb manufacturing, when systems and platforms that are standard today (e.g., suspension-capable cell cultures and single-use systems) faced considerable skepticism. Each successive wave of development provides manufacturers and regulators with an opportunity to reassess the risks involved, observe the advantages, and acclimate to the new and inevitable changes in manufacturing.
The first wave of multimodal manufacturing already is here. It brought with it the concept of operating dedicated suites concurrently to produce multiple therapies inside the same facility. The second wave will carry us even further — right into using individual good manufacturing practice (GMP) suites that can be engineered to campaign between products smoothly and efficiently.
When we asked survey respondents about that evolution, 69% said that they “valued” or “highly valued” the concept of suite-level multimodal manufacturing. Some of those respondents, however, stressed the importance of first understanding the risks involved. That is where the lessons learned from multimodal manufacturing at the facility level are instrumental. By examining those, we can better understand how to control risks and maximize opportunities of bringing multimodal manufacturing into production suites. Our CRB specialists are deeply involved with this evaluation, and they have identified four key areas on which the future of suite-level multimodal manufacturing depends for cell and gene therapy companies. Those areas are facility flows, utilities, cleaning and decontamination, and equipment turnover and validation.
Facility Flows
Cell and gene manufacturers need systems designed for continuous segregation. For a multimodal facility that operates dedicated suites in parallel, a detailed understanding of production risk profiles is key. Process closure isn’t enough. Manufacturers should develop strategies that ensure process segregation both within a given modality and between different modalities across a facility. That can be completed primarily by implementing physical segregation; appropriate heating, ventilation, and air conditioning (HVAC) systems; high-efficiency particulate-absorbing (HEPA) filtration; and airlocking and pressurization; and so on.
In a multimodal facility, flow of personnel and support materials can change as individual suites campaign between modalities. Whether a badge system or other mechanism for controlling access is in place, operators need to be able to reconfigure those systems based on the product combination being manufactured. Signage provides another layer of protection against the risk of cross-contamination. For example, a light indicator system could include different colors that correspond with different modalities, indicating which materials can be transitioned into a manufacturing suite. Wherever possible, carts, totes, cleaning equipment, solutions, and other support components should be dedicated to specific products or areas and/or be disposed of after a single use.
A facility that is operating multimodal suites has greater risks of cross-contamination than one that does not. The facility’s segregation strategy must stand up to high scrutiny. Manufacturers must build on the standardized precautions described above to identify and prevent potential worst-case scenarios. Process closure is critical. Redundant systems should ensure the continuous operation of exhaust fans, fan arrays for cleanroom HVAC, standby power, uninterruptible power supply (UPS), and proper shutdown sequencing.
Unidirectional process flow is critical in facilities operating multimodal suites. For example, such facilities will need a strategy for transporting waste (a critical product-differentiated material) without the possibility of contacting other product-specific components. Quality control (QC) cross-product sample testing needs a documented risk assessment, and tests with high-risk profiles might require dedicated testing spaces.
 Utilities
Utilities
A utility strategy should be flexible and cost-effective. Utility systems in a multimodal facility operating dedicated suites need robust risk profiles to ensure that water, gases, and clean compressed air are distributed as required without the risk of cross-contamination.
For example, several potential design solutions can be used for distributing water for injection (WFI), including single-loop systems and dedicated loops for each suite or each group of modalities. Manufacturers should consider the risk profiles of different options when designing systems that provide the required level of protection against cross-contamination. Such considerations also apply to systems that supply gases and air to support different processes. Cell and gene manufacturers should design these systems based on case-by-case risk assessment, factoring in every element of a system’s segregation. For example, if a suite uses bulk tanks with separate piping systems instead of dedicated gas cylinders, manufacturers need to choose a location for downstream filters that will keep use points adequately separated.
When product-agnostic manufacturing suites come into play, utility systems bear the additional responsibility of supporting frequent product changeovers. To balance the need for flexible utility systems with the need to control costs, manufacturers should have two resources available: good design expertise and a good understanding of where true risks lie. For example, a lean approach might mean having fewer WFI drops and dispensing more volume in just a few solution-preparation suites dedicated to compatible modalities. Whatever the solution, a robust control strategy based on appropriate monitoring and rapid detection of all potential contaminants will be critical.
Cleaning and Decontamination
Multimodal facilities need protocols designed to create product-neutral areas. Whether operating a multimodal facility with dedicated suites or one with suites designed to campaign between process platforms, a biomanufacturer should assess every process component and material to formulate valid and repeatable cleaning standard operating procedures (SOPs). Cleaning personnel and their equipment and materials should move in and out of performance along segregated facility pathways. Operators also should have control systems in place for rapid identification, tracing, and isolation of contamination incidents.
Manufacturers who are campaigning their suites between modalities face the additional burden of returning their manufacturing spaces to “product neutral” between campaigns. Cleaning and decontamination procedures need to reflect that need. Between different modalities, a full-room fumigation typically can achieve the level of decontamination assurance. A chemical wipedown also might be appropriate between different batches of the same product, depending on the established risk profile associated with a given modality.
Equipment Turnover and Validation
Cell and gene manufacturers need to be able to move equipment in and out of use smoothly and reliably. Unlike multimodal manufacturers with dedicated suites, companies that campaign their suites between modalities will need to disassemble and reassemble their manufacturing trains regularly.
That challenge of equipment turnover is not new, and it needn’t be an intimidating one. Contract manufacturing organizations (CMOs) that have frequent changeovers between clients have solved that problem. The key is to design each manufacturing space with equipment needs in mind. Operators will need to return equipment to a validated and consistent state of performance between changeovers. Operators also need well-documented procedures for dismantling, storing, reassembling, and calibrating that equipment. That applies to everything from the layout of a suite to the ergonomics of its equipment and the travel pathways available for moving equipment in and out of performance (e.g., door sizes, ramps, and the turning radii around corners).
Manufacturers must consider all of those details when planning their multimodal suite layouts. The bottom line is that everything must be connected properly and functioning predictably according to robust and streamlined performance requalification procedures.
A Breaking Point Is Coming
It might take time for the industry to adjust to the concept of multimodal manufacturing at the suite level, just as it took time to assess and accept previous technological advances in cell and gene therapy manufacturing. A breaking point is coming, whether the industry is ready or not, and solutions will be needed when it does.
Turn-key, product-neutral GMP suites should be designed for flexibility and product integrity. To get to that point, cell and gene therapy manufacturers can learn from the larger biopharmaceutical industry’s first explorations of facility-level multimodal manufacturing and extend those lessons into the future, thereby identifying and planning for the needs of an intensely agile, multiproduct manufacturing solution.
Corresponding author Peter Walters is director of global advanced therapies at CRB in San Marcos, CA; [email protected]. Noel Maestre is vice president of life sciences at CRB and cofounder of SlateXpace in Carlsbad, CA; https://www.slatexpace.com.
You May Also Like