Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
The increasing application of single-use components and systems in bioprocessing represents one of the most significant changes in biopharmaceutical manufacturing in recent times. Driven by various factors such as improved efficiency, flexibility, and economics, this trend also presents specific challenges to end users. In one industry review by Langer, extractables and leachable compounds from disposable components were considered by end users to be a major area of potential concern regarding safety, efficacy, and stability of the pharmaceutical product (1). In a more recent survey by the Bio-Process Systems Alliance (BPSA), however, extractables and leachable compounds were considered by only 13% of respondents to be a barrier in integrating single-use technologies into existing or new processes (2). The apparent change in thinking may be due, in part, to an increased amount of information available in published case studies, reviews, and industry guides such as those issued by BPSA (3,4) covering regulatory issues, risk assessment, and test programs.
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: UPSTREAM AND DOWNSTREAM PROCESSING
WHO SHOULD READ: PRODUCT/PROCESS DEVELOPMENT QA/QC, AND ANALYTICAL
KEYWORDS: ANALYTICAL METHODS, PROCESS VALIDATION, DISPOSABLES
LEVEL: INTERMEDIATE
Extractables are typically determined with laboratory tests using standard extraction fluids termed model solvents. Such tests are designed to exceed worst-case product and process conditions and reveal chemical entities that may migrate from process components into the final product as potential leachables.
The quality and quantity of materials extracted from an organic polymer component depends not only on the materials of construction, but also on the contact fluid composition, temperature, contact area, and contact time. In a multicomponent single-use system, the importance of each parameter in the migration of potential leachable compounds will vary according to component type and process conditions. For example, a sterile connector may have only transient exposure to a fluid during transfer, whereas a flexible biocontainer may be in contact with product or process fluid for many hours, days, or even months. Similarly, a typical small capsule filter with an effective filtration area of 0.15 m2 may have an internal surface area of ~150 m2 compared with a 0.5-L flexible biocontainer with a surface area of only 0.05 m2 — 3,000× smaller. For these reasons, extractables studies should be designed and performed under conditions appropriate for each specific component and process application. That can provide an accurate and suitable assessment of the potential for the component or system, to release leachables into the drug product.

We applied this customized approach to two previous studies on extractables In the first study, we reported extractables results from tests on sterile connectors and membrane filter capsules (5). The test articles were preconditioned by gamma irradiation at 50 kGy to represent worst-case sterilization conditions and extracted under exaggerated simulated conditions using a recirculating system with extraction times of four hours for ethanol and 24 h for deionized (DI)water. Qualitative and quantitative extractables results on sterile connectors using 13 analytical methods showed very low levels of extractables, mostly below the limit of detection (LOD) or no different from the controls. We concluded that the potential for these connectors to release leachable materials into compatible drug products was very low. For the capsule filters, higher levels of extractables were obtained, as expected from the high internal surface area of the microporous membrane within the filter. Qualitative analysis showed that all compounds detected were consistent with the filter’s materials of construction, which passed biological safety tests such as USP “Biological Reactivity, in Vivo, for Class VI Plastics.”
Table 1: Single-use components in a test system
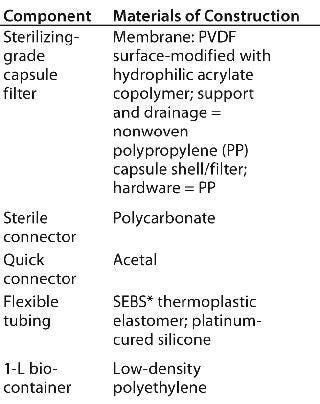
Table 1: Single-use components in a test system ()
In the second study, we characterized extractables from thermoplastic tubing and flexible biocontainers (6). For the tubing, a worst case was represented by presterilizing with gamma irradiation at 50 kGy and agitating the test fluid of ethanol or DI water within clamped segments of tubing for 72 h. Very low levels of extractables were detected, many below the limit of quantification (LOQ). For the biocontainers, a worst case was simulated by filling with test fluid and storing at 40 °C for 30 days. Results showed very few organic compounds above the LOD for water extracts and those identified were in the low parts per million (ppm) or parts per billion (ppb) range. Ethanol extracts gave some additional identifiable compounds, which were mostly oligomers of the base polymers or degradation products of the antioxidants used in the polymer formulations.
Here we report on extractables studies performed on a total system containing all four components previously tested: capsule filter, sterile connectors, plastic tubing, and flexible biocontainers. The test protocol was designed to simulate a typical operating procedure for this type of single-use system and under typical worst-case conditions.
Objectives
We set out to meet four objectives
To quantify and characterize extractables from a model multicomponent, single-use system.
To study the effect of system flushing
To assess the effect of storage and extraction times of up to one year in biocontainers
To develop a method of determining extractable and potential leachable compounds for process validation of single-use processes in biopharmaceutical manufacturing.
Experiment
Our test system included three separate segments: fluid supply system, filter manifold, and biocontainer manifold (Figure 1). The fluid supply system provided clean extraction fluid for the single-use system manifolds and a sampling point for negative controls. To maintain the extraction fluides at highest purity, we used a polytetrafluoroethylene (PTFE) diaphragm pump and tubing with a glass reservoir. The filter manifold contained identical lengths of flexible tubing between the filter and the final sterile connector to ensure the same cont
act area and extraction conditions.
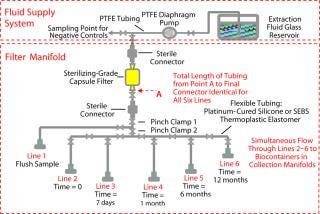
Figure 1: ()
Each line was connected to a biocontainer manifold (Figure 2). Line 1 was used for the system flush sample, and the other five lines were used for extractables time tests from zero to 12 months. The biocontainer manifold was designed to collect 2 L of extraction fluid using 2-L × 1-L biocontainer bags. The test manifolds were gamma irradiated at 50 kGy and all extractables tests (including biocontainer storage) were performed at 21 °C. Test solvents were 18 MΩ-cm DI water and 100% ethanol HPLC grade. The complete study was performed in duplicate.
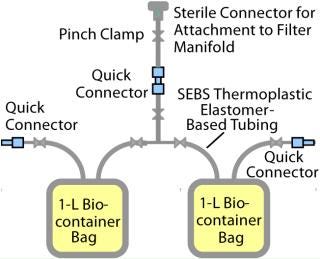
Figure 2: ()
The main components in this model single-use test system were the same as those used in our previous extractables studies on the individual components (5,6). Table 1 shows further details.
Test Procedure:Figure 3 summarizes the major stages of the test procedure.
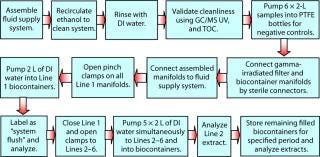
Figure 3: ()
Analytical Methods: We performed qualitative and quantitative analyses on the extracts using 13 methods and reported details about these methods in previous studies (5,6). Table 2 lists our analyses on water and ethanol extracts.
Table 2: Analytical methods used for assessment of extractables
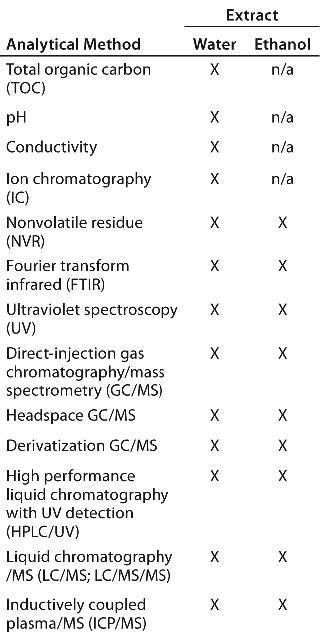
Table 2: Analytical methods used for assessment of extractables ()
Results and Discussion
Because of space restrictions, we can’t report here our detailed results (information available from Pall Life Sciences on request). However, the main and the most significant results of the extractables analyses are presented here, which can be conveniently divided into three groups.
Group 1 — TOC, Conductivity, pH, and Ion Chromatography: We performed these analyses on water extracts only (Table 3).
Table 3: TOC, conductivity, pH and ions in water extracts1
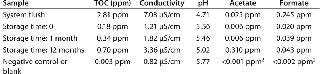
Table 3: TOC, conductivity, pH and ions in water extracts1 ()
TOC: TOC levels, after a system flush, were very low at
In our previous extractables studies, we established that the bulk of the extractables were derived from the high-area filter membrane (5). Those results suggest that best practice for lowest leachable compounds is to perform an initial preflush, whenever possible, of either the full system or the filter, and to discard the flushed liquid.
Conductivity: The conductivity values showed, after system flush, only a small increase over negative control and a slow rise in values over the 12 month storage and extraction period.
pH: After the system flush, the pH values showed a shift of 0.21 units at zero time rising to 0.75 units after 12 months. These shifts are within one pH unit and in compliance with US Pharmacopoeia requirements.
Ion Chromatography: We used ion chromatography to measure acetate and formate levels. Both compounds can be found in small quantities in most plastics and may come from the raw materials, but they are also the smallest breakdown products of larger molecules and can be a strong indicator of chemical instability of a component, especially under extreme conditions. All extracts, including the system flush sample and the 12 months storage sample, showed levels
Group 2 — Nonvolatile Residues (NVR) and Fourier Transform Infrared (FTIR) Spectroscopy: NVR analyses provide a quantitative measure of low volatility extractables. Dried residues (obtained after evaporation of test solvent) from the extract can then be analysed qualitatively by FTIR to identify specific functional groups and differences between test extracts. Using the results of NVR in water and ethanol extracts (Table 4) we can make several conclusions.
Table 4: Nonvolatile residues in extracts1

Table 4: Nonvolatile residues in extracts1 ()
First, the NVR level in the flush sample for DI water of 1.9 mg is relatively low compared with the ethanol extract of 119 mg. That is typical of extractables data on single-use components because ethyl alcohol is a more aggressive solvent, especially for additives and residual oligomers in organic polymers.
Second, results show a time-dependent, gradual increase in extractables for both water and ethanol. That indicates a continuous but slow leaching of extractables from the polyethylene biocontainer, the only component in contact with the fluid during storage.
Figure 4 shows FTIR spectra of residues in the ethanol extract from the system flush and the extracts after 12 months storage. The major difference between spectra is the presence of some peaks (circled) in the system-flush sample, which are not present in the extracts stored for 12 months. This result indicates that these compounds can be easily removed by system flushing to minimize extractables. The absence of additional peaks after storage indicates a relatively stable environment during storage with no significant extraction of additional nonvolatile compounds or release of nonvolatile breakdown products.
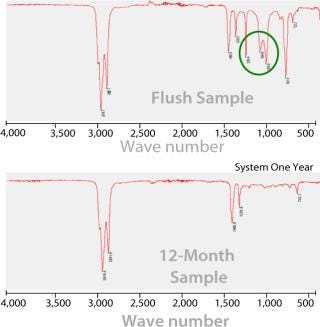
Figure 4: ()
Group 3 — GC/MS, LC/UV/MS, and ICP/MS: These methods are powerful tools for identifying a range of organic compounds and inorganic elements in extraction fluids and can quantify them down to ppm, ppb, and, in some methods, ppt (parts per trillion) levels.GC/MS Analyses: We applied the following analytical strategies for GC/MS when investigating trace levels of extractables:
Concentrate extracts to maximize detection
Carefully interpret MS data
Use authentic standards
Match the mass spectra and retention times
Ensure accurate calibration and quantification
Perform recovery studies.
Results show no detectable peaks in water samples for volatile and semivolatile compounds in both the flush sample and after 12 months storage and extraction (Table 5). Derivatization results for organic fatty acids identified only three acids, all
Table 5: Identity of compounds in deionized water (DI) and ethanol extracts by GC/MSanalysis
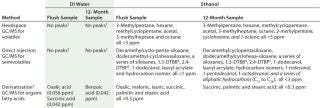
Table 5: Identity of compounds in deionized water (DI) and ethanol extracts by GC/MSanalysis ()
Because most pharmaceutical products are water-based, GC/MS results indicated very low extractables for these types of compounds can be expected for this type of multicomponent single-use system. The ethanol extracts revealed several identifiable volatile and semivolatile compounds in the flush sample (Table 5) and some additional compounds (shown in italics) after 12 months of storage and extraction. The data demonstrate a much stronger solvent action of ethanol. However, all compounds can be traced back to the individual single-use components in the test system, as reported previously (5,6). Furthermore, all were present at a low concentration of
LC/UV/MS analyses are ideal for detecting trace levels of antioxidants and their degradation products, as well as heat-sensitive organics in extraction fluids. An analytical strategy to achieve precise identification and highest possible resolution must include careful evaluation and interpretation of MS data, use of authentic standards, matching mass spectra and retention times, and precise calibration and quantification.
Analysis of water samples by LC/UV/MS showed no peaks at LOD between 0.1 ppm and 1 ppm using a mixture of antioxidants as standards. For the ethanol extracts, the compounds identified included 1,3-ditertbutylbenzene (1,3-DTBB) and 2,4-ditertbutyl phenol (2,4-DTBP), which we identified in previous studies as degradation products from polymer additive (Irgafos 168 phosphate) in specific single-use components of the test system (5,6).
ICP/MS Analyses: Inorganic elements, especially heavy metals, can influence the efficacy, safety, and stability of some drug products. Analysis with ICP/MS provides a convenient and highly sensitive method for identifying and quantifying a wide range of metallic ions.
The ethanol extracts showed no elements higher than the LOD or LOQ, except for calcium at 5.9 ppb (Table 6). For water extracts, nine elements were detected in the flush sample at low ppb concentrations.
Table 6: Elements in water and ethanol extracts
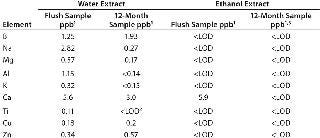
Table 6: Elements in water and ethanol extracts ()
The 12-month sample showed reduced levels, mostly
Discussion and Conclusions
In this study, we generated additional information about extractables from single-use systems that could impact the qualification and validation of biomanufacturing processes. In our previous studies, we took one type of component (e.g., a capsule filter or a biocontainer) and designed a specific extractables procedure to represent a worst-case condition for that component (e.g., continuous recirculation of extraction fluid through a filter or storage of filled biocontainers at 40 °C for 30 days). In this way, a worst-case extractables profile (qualitative and q
uantitative) could be established for each component. This data library facilitates selection and qualification components for single-use systems.
Furthermore, during process validation, if such data from all components of single-use system are available and the test conditions represent a worst case to process conditions, then little or no further extractables testing may be required. Otherwise, a process- and product-specific extractables validation study may need to be performed on the whole single-use system for determining potential leachables and assess any toxicity or impact on quality of a final drug product.
The study results for components (5,6) and model single-use system (this article) can facilitate process- and product-specific extraction tests (potential leachables) using model solvent as well as process fluid because the potential leachable compounds (at least the majority of them) are already identified. Furthermore, the effect of preflushing the system could provide supporting data for preparing suitable standard operating procedures (SOPs) to minimize leachable compounds.
From the results, we showed:
It is feasible to perform extractable studies on complete single-use systems.
A short system flush can remove a substantial proportion of extractable material.
Water extractables were substantially lower than ethanol extractables for organic compounds.
In most cases, no significant increase in extractables was seen after storage for up to 12 months.
Identified compounds were also found in previous studies on the individual components.
Those compounds could be traced to the materials of construction of single-use components.
With advanced analytics, a wide range of compounds can be identified and quantified. Such characterization is ideal and suitable for analyzing extractables from single-use systems.
We conclude that a suitable method for extractables testing of multicomponent single-use systems has been developed and qualified. The data generated can also be available and of benefit to end users in their qualification and validation of similar bioprocesses.
About the Author
Author Details
Weibing Ding is technical manager at Pall Life Sciences Scientific and Laboratory Services, David B. Pall Technical Center, 25 Harbor Park Drive, Port Washington, NY 11050; 1-516-801-9254, [email protected]. Jerold Martin is senior vice president of scientific affairs at Pall Life Sciences; 25 Harbor Park Drive, Port Washington, NY 11050; 251-516-801-9086, fax 1-516-484-5228; [email protected]; www.pall.com
1.) Langer, E. 2007.Advances in Large-Scale Pharmaceutical Manufacturing2nd Edition, BioPlan Associates Inc, Rockville.
2.) Caine, B. 2009. BPSA Survey: The Impact of Single-Use Technologies, Bio-Process Systems Alliance.
3.) Colton, R. 2008. Extractables and Leachables Subcommittee of the Bio-Process Systems Alliance. Recommendations for Extractables and Leachables Testing. BioProcess Int 6:S28-S39.
4.) Martin, J. 2010. Recommendation for Testing and Evaluation of Extractables from Single-Use Process Equipment, Bio-Process Systems Alliance.
5.) Ding, W, and J. Martin. 2008. Implementing Single-Use Technology in Biopharmaceutical Manufacturing: An Approach to Extractables/Leachables Studies, Part One — Connectors and Filters. BioProcess Int 6:34-42.
6.) Ding, W, and J. Martin. 2009. Implementing Single-Use Technology in Biopharmaceutical Manufacturing: An Approach to Extractables/Leachables Studies, Part Two — Tubing and Biocontainers. BioProcess Int 7:46-51.
You May Also Like