Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Single-use (disposable) technologies are gaining significant traction in biopharmaceutical manufacturing due to reductions in capital investment for plant construction, lower requirements for cleaning and sterilization, and the advantages of eliminating cross-contamination during multiproduct manufacturing (1,2,3,4). In the early days of disposables, single-use (SU) systems were used only in specific unit operations (5, 6). Recently, however, options have become more widely available throughout drug-substance manufacturing (7,8,9,10). Companies now focus on selecting the right SU technology from an array of options and integrating those choices into production processes.
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: Manufacturing
WHO SHOULD READ: MANUFACTURING, PROCESS DEVELOPMENT, facilities
KEYWORDS: DISPOSABLES, bioreactors, chromatography, filtration, inoculum, centrifugation, storage, freeze–thaw
LEVEL: Intermediate
KBI Biopharma operates an end-to-end current good manufacturing practice (CGMP) manufacturing facility for biologics derived from mammalian cell culture up to the 2,000-L scale. That experience has given us a unique vantage point into disposables. Here we sample the diversity of SU technologies available for each unit operation in a biomanufacturing process and highlight some key considerations for integrating them. One remaining concern has to do with scaling up a cell culture process to achieve comparable performance at different scales, so we illustrate a successful scale-up using disposable bioreactors.
Growth of SU Technologies for Bioprocessing
Disposable shake flasks, roller bottles, pipettes, and filters have long been used in cell culture laboratories and at the inoculum stages of large-scale processes (2). The first introduction of SU technology for larger-scale cell culture operations came with introduction of the Wave Bioreactor system in 1996 (11). It uses waves in a rocking bag containing cell culture medium to provide agitation and aeration for cell growth. As time went on, that system established itself for inoculum trains in large-scale cell culture and as a production system for smaller-scale cultures (12). That concept evolved into the first stirred-tank SU bioreactor for mammalian cell culture with a 250-L unit launched in 2004 (13). Subsequent years have brought larger scales of such bioreactors (1,000 L in 2006, 2,000 L in 2009) while expanding the number of vendors for such technologies (14).

Figure 1: ()
In downstream processing, all depth, membrane, and viral filters were SU devices even when their housings were reused. Such systems gradually expanded to include commercially available membrane chromatography devices (in the late 1990s) and containers (bottles and bags) to store drug substances and process intermediates. GE Healthcare introduced its ÄKTA Ready system with a fully disposable fluid path in 2009. The past five years have seen an explosion in the number and variety of SU technologies and the types of unit operations they now cover (chromatography, ultra-and diafiltration, centrifugation) (2, 15). An end-to-end protein manufacturing set-up exclusively based on disposable systems is now possible (1, 7, 16), as is the case with KBI Biopharma’s 2000-L CGMP manufacturing area. But a more common choice is to configure these systems to expand the capacity and flexibility of conventional, hard-piped, stainless steel facilities.
A number of factors drive the mainstream adoption of SU systems. A key advantage comes in eliminating the need for clean-in-place (CIP) or steam-in-place (SIP) systems in biomanufacturing facilities. Single-use systems are usually gamma-irradiated by their suppliers, a process that eliminates the need for cleaning to prevent cross-contamination in multiproduct manufacturing facilities or contamination by adventitious agents such as microbes. That in turn reduces the scale of utilities needed to support bioprocessing plants, which can significantly reduce the square footage of such facilities and thus their cost of construction (17). It has been estimated that up to 40% capital cost reduction is possible for facilities using SU systems compared with conventional facilities (16, 18).
The reduced requirement for cleaning and sterilization leads some to conclude that disposables are “greener” (19). It appears that limitations in the scale of production bioreactors to 2,000 L is likely one main hurdle that still restricts the adoption of SU systems for commercial biopharmaceutical production. Additional considerations that disposable systems bring to the fore include a need for more extensive leachables and extractables studies (20). Although advantages of SU systems are dealt with elsewhere (10, 13), here we provide a synopsis of such technologies currently available for bioprocessing and discuss some of their key features.
Figure 1 is a schematic of a typical biomanufacturing process using disposables for all unit operations. It highlights the diverse range of unit operations that go into producing a biopharmaceutical drug substance. Production typically begins with thawing a single vial of cells and increasing cell counts through inoculum expansion. That process at early stages typically uses shake flasks or spinner flasks before progressing to larger-volume cultures through wave-type or other small-scale bioreactors. Combining wave-type and seed bioreactors is typical in mammalian cell culture seed trains.
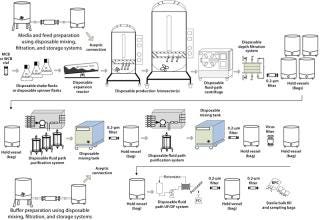
Figure 1: ()
The seed bioreactor is used to inoculate a production bioreactor, in which cells shift from a growth phase to a product-expression phase during which a protein of interest is secreted into the cell culture medium. Harvest and clarification remove cells and cell debris to produce a process fluid that can be loaded
onto chromatography columns for purification. Typically a centrifugation step is followed by depth and membrane filtration to remove cells and cell debris. For smaller cell cultures, a process using depth filtration for primary harvest and clarification often can be used.
Downstream processing is intended to produce a drug substance with consistent purity that is compatible with clinical dosing in humans. This is achieved through a combination of column chromatography operations along with membrane chromatography, buffer exchange using ultra-and diafiltration (UF/DF), and viral filtration steps.
Throughout manufacturing, ancillary unit operations include buffer and media batching in mixing vessels, storage/hold of process intermediates between steps, and membrane filtration to augment bioburden control. A number of connections need to be made to transfer process intermediates from one manufacturing stage to another. Sensors and detectors are used throughout.
Finally, a purified drug substance is transferred to appropriate containers for storage and shipping. To base a facility on SU technology, all unit operations need viable alternatives that enable successful operation with disposable product-contact surfaces. Below we detail those unit operations and describe the current state-of-the-art, including challenges that remain to be addressed.
Upstream Processing
Table 1 lists some vendors that offer single-use systems for upstream processing.
Table 1: Single-use options available for upstream and downstream unit operations*
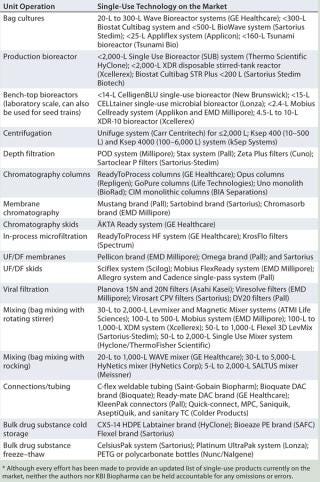
Table 1: Single-use options available for upstream and downstream unit operations* ()
Early Inoculum (Vial Thaw to Shake Flask/Spinner Cultures): The first process steps are traditionally operated using disposables. Steps from vial thaw to cell expansion involving shake flasks, T-flasks, spinner cultures, and roller bottles are well established in bioprocessing. Spinner cultures use special flasks with hanging stir bars or vertical impellers that provide mixing and aeration. Such cultures work with 100-mL to 500-mL volumes.
Cells can also be cultured in simple flasks of culture medium with rotary agitation by a shaker platform for mixing and aeration in a simple system. Shake flasks are highly versatile. Some groups have developed acceptable models of bioreactor operations with shake-flask systems by controlling their external environment with incubators as well as controlling their agitation rates and fill volumes. Feeds can be introduced manually in a sterile environment. Shake flasks can be operated ≤3 L in volume in parallel, making this a versatile system for the early inoculum stages of a process.
Inoculum Train: The middle steps in an inoculum process further expand cell counts at 2-to 200-L volumes. A number of SU technologies have established themselves at this stage as well. Prominent among them is the WAVE Bioreactor system, which was one of the first SU technologies applied to larger-volume cell cultures (11). It rocks a bag of cell culture medium on a platform to agitate the culture and provide aeration from the bag headspace. Rocking rate, rocker angle, and bag fill volume influence a culture’s oxygen transfer rate. Design improvements enable fed-batch and even perfusion cultures to be operated using such bioreactors. A number of manufacturers have launched agitated-bag designs for SU bioreactors (Table 1). Given the range of culture volumes that are possible with this kind of set-up, it has been widely applied to production of relatively smaller product quantities (12).
Stirred-tank bioreactors are used both in late seed-train stages and production cultures. Stirred-tank SU bioreactors are currently available in volumes up to 2,000 L. Bench-top sizes come from a variety of vendors (Table 1).
For larger volumes, two primary platforms are commercially available. The Thermo Scientific HyClone Single Use Bioreactor (SUB) system was the first large-scale platform. Xcellerex then launched its XDR disposable stirred-tank bioreactor. Both systems use an angular, axial flow impeller that is off centered. That placement eliminates the fluid vortex that otherwise forms in stainless steel bioreactors, making baffles unnecessary. The Xcellerex bioreactor has a magnetically coupled, bottom-driven impeller; the HyClone system has a top-driven impeller. Both are well established at their respective scales, with a few published studies successfully comparing their data from those generated using conventional bioreactors (21). Below we offer more data on scalability using disposable bioreactor systems. The high level of interest in these systems is reflected in GE Healthcare’s March 2012 decision to purchase Xcellerex.
Sensor technology is still evolving for single-use bioreactors. A typical mammalian cell culture operation combines in-line and off-line measurements for appropriate monitoring and control.
Off-line measurements are conducted through aseptic withdrawal of samples from a bioreactor — analogous to those conducted with conventional formats. Cellexus Biosystems markets a sampling system that is connected to a bioreactor with a presterilized Luer connection incorporating a one-way valve for collection into sample pouches. For a new aseptic connection, users can aseptically weld to the bag system using thermoplastic tubing and a tube welder (e.g., from Terumo Medical Corporation).
Direct in-line sensors typically measure pH, temperature, dissolved oxygen (DO), and carbon dioxide (CO2). Sensors that directly contact a culture must either be sterilized by autoclaving or be disposable themselves (integral to a bioreactor bag) and sterilized by the same gamma irradiation used to ensure sterility for the bag assembly.
An area of further development is in optical sensing for disposable bioreactors to make in-line measurements through transparent viewing windows in bioreactor bags. Continued progress in sensor technology is important for application of process analytical technology (PAT) in single-use bioreactor systems. That could include an extension of additional in-line measurements such as infrared or Raman spectroscopy and electrochemical or optical detection.
Cell Culture Harvest and Clarification
Mammalian cell cultures are typically harvested and clarified through tandem centrifugation, depth filtration, and membrane filtration steps (12).
Centrifugation has been a late entrant to the single-use technology space. Nevertheless, two different single-use centrifugation technologies are now on the market. The kSep centrifuges (kSep400 and kSep6000) from kSep Systems are low-shear, single-use centrifuges that handle 1-L to 6,000-L bioreactor volumes. Advantages include robust scalability, recovery of product from the interstitial spaces between cells (washing) without significant dilution, clarification and discharge at constant RPM, and fully automated processing. These systems have been successfully implemented for applications in harvest clarification as well as for processes in which cells are the product (cell therapy) or an intermediate product (vaccine manufacturing). Traditional centrifugation systems (including disk-sta
ck centrifuges) cannot be used for high-density fed-batch processes because frequent discharge leads to significant loss of product. Implementations of kSep centrifuges have provided >99% product recoveries by introducing a quick wash sequence.
Another option (Carr Centritech’s Unifuge system) is based on a rotating tubular-bowl design with an integrated disposable fluid path. This system is designed for low to intermediate scales, with flow rates of 0.1–4 L/min, and is stopped between cycles for emptying. Given the inherent limitations in throughput for the tubular bowl design (which led to the advent of the disk-stack centrifuge), additional development probably will be needed for this system to be directly scaled up for large-scale mammalian cell culture operations.
Depth filters trap particulates in the matrix of a fibrous bed that has a tortuous flow path. At smaller scales (≤2,000 L), depth filtration is often used as the primary cell-removal step for mammalian cell culture operations. The fibrous bed includes a binder for depth-filter matrix rigidity. It also serves to hold adsorptive material such as diatomaceous earth that confers additional adsorptive properties (23).
Depth filtration is traditionally a single-use operation (24, 25). Traditional designs, however, stacked into housings (which required SIP during manufacturing) to offer the surface area needed for large-scale harvest operations. Millipore (now EMD Millipore) was the first vendor to market a depth filter housed in a plastic holder (the Millistak+ POD system) that eliminated the need for a separate housing. The company now offers 23 depth filtration media in the POD format, and they can be linearly scaled as required by attaching more filters using expandable holders. Surface areas for each filter unit range from 0.027 m2 for laboratory “scale-down” filters to 1.4 m2 for large-scale systems. Up to 10 POD units can be stacked per rack, with up to three racks creating an assembly with 52 m2 of available surface area for filtration ≤12,000-L bioreactor scale.
The Stax system from Pall uses Seitz depth-filter media with filter areas ranging 0.25–2.00 m2 per unit. The stackable design is similar to Millipore’s, permitting linear addition of filter surface area for large-scale operations. Sartorius-Stedim Biotech’s Sartoclear P depth-filtration system comes in 10-in. and 20-in. diameter MaxiCap units or S9P 12-in. and 16-in. sizes that are also stackable for large-scale operation. And 3M markets Cuno Encapsulated Zeta Plus depth-filtration systems.
A few case study reports have attempted to compare those systems for mammalian cell culture harvest (26), but selection is usually made based on manufacturing experience with operation of each large-scale platform as well as media performance during laboratory-scale testing for clarification efficiency. To select the best depth filter and surface area for harvest and clarification operations, process designers need to remember that such filters have adsorptive properties. Very often, that selection process also involves testing subsequent membrane filters to compare the clarification performance of the depth filters.
Membrane Filtration: Membrane microfilters (0.10–0.45 μm) have been single-use systems even in conventional stainless steel manufacturing plants. These are used extensively throughout bioprocess operations for applications ranging from buffer and media filtration to serving as column guards and in-process filters to ensure bioburden control before process hold steps.
Downstream Processing
Disposable Chromatography Columns: Chromatography is the workhorse of downstream separation processes for most biopharmaceutical manufacturing. The range of resin chemistries and modes of operation have expanded greatly over the past couple of decades, presenting separations scientists with a broad range of choices. Companies want to use conventional preparative chromatography resins in single-use operations. For example, KBI Biopharma’s SU cell culture facility dedicates columns and resins for a given product and process but reuses the media for multiple cycles of the same product. The benefit of using established chromatographic resins and the relatively high cost of those resins (if treated as consumables) drive this operating paradigm. But several vendors have introduced SU columns and resins.
The most widely used disposable columns are offered by GE Healthcare: ReadyToProcess go up to 20 L in column volume and come prepacked and presanitized. SU columns can be fairly expensive (considering the costs of both columns and media). If they are used for clinical manufacturing, however, they can be cycled extensively per batch to reduce cost. GE Healthcare can pack any of its commercial preparative chromatography resins in ReadyToProcess columns. The Opus series from Repligen goes up to 30 cm in column diameter, with an extension to 60-cm diameter columns expeted soon. Repligen can pack any preparative chromatography resin in Opus prepacked columns. And the GoPure series from Life Technologies extends to the 8-cm diameter for POROS chromatography resins.
In monolithic columns, the entire bed is a single integral polymeric shape rather than formed by the smaller beads packed into typical columns. Monoliths have established themselves in analytical chromatography. However, despite early interest, they have faced difficulty in process-scale chromatography. But these resins have made a comeback due to interest in single-use chromatography. Both Bio-Rad Laboratories and BIA Separations sell monolithic media.
Chromatography Skids: The ÄKTA Ready chromatography skid from GE Healthcare is widely used in SU manufacturing settings. It offers a fully disposable fluid path both to and from its chromatographic columns and can handle pressure ≤5 bars. The system helps companies eliminate the need for cleaning procedures for their processing equipment between batches.
Membrane chromatography elicited significant interest in the early 1990s because it combined highly selective ligand interactions with the high throughput of membrane filtration systems. This technology faced a setback then as attempts to obtain very high binding capacities per membrane volume unit failed (in comparison with chromatographic resins). But over the past decade, membrane chromatography has reemerged, offering process engineers the chance to conduct operations using a high-capacity flow-through mode in which a protein of interest flows through while impurities bind to the membrane surface.
In particular, anion-exchange (AEX) membranes for monoclonal antibody (MAb) purification (because MAbs are typically basic proteins) has established itself as a polishing step wherever low levels of impurities such as DNA or endotoxins need to be cleared or an additional viral clearance step is required. Several vendors sell AEX membrane adsorbers: Pall’s Mustang series (≤5 L membrane volume), Sartorius Stedim Biotech’s Sartobind series (≤5 L volume), and Millipore’s Chromasorb series. Those systems are typically housed in contained modules and operated as SU products. Several come with alternative modalities such as cation-exchange (CEX) Mustang and Sartobind membranes; hydrophobic-interaction chromatography (HIC) Phenyl membranes from Sartorius Stedim Biotech; and AEX with a salt-tolerant ligand that changes its conformation based on ionic strength, to permit operation at high salt concentrations (Sartorius STIC and Millipore Chromasorb Q membranes).
Viral filtration traditionally involves SU membrane systems. Table 1 lists some leading types of parvoviral-grade filters that remove potential viral contaminants based on size. As in conventional facilities, the surface area of these high-cost filters needs to be optimized in SU operations to use the
minimum surface area for robust operation.
Tangential-flow filtration (TFF) is commonly used for buffer exchange based on UF/DF. Currently, single-use membrane surface areas are relatively restricted, with 2.5 m2 as their upper limit. Disposable–flow-path TFF skids are available from Scilog (Sciflex 150-TFF), EMD Millipore (Mobius Flexready for TFF), Pall (Allegro), and Sartorius (Sartoflow Alpha Plus SU). The latter are currently limited in membrane surface area to ≤0.3 m2. These systems are compatible with standard cassette-held UF/DF membranes. And the Pall Cadence single-pass TFF system (using Pall Delta membranes) conducts TFF with only a single pass of fluid, eliminating the need for recirculation.
Tanks and Mixing Technologies: Tanks lined with single-use bags are used for collecting column eluates and other in-process intermediates throughout downstream processing (27). Very often, process intermediates are filtered through a microfiltration membrane into these bags for bioburden control. Large-volume buffer preparation also requires bag mixing systems. As Table 1 shows, a number of single-use mixing systems with rotating stirrers are available in volumes ≤2,000 L. Other types come as bag systems with oscillating devices for mixing.
Drug Substance Freeze–Thaw: After purification, drug substances are either stored at 2–8°C or frozen, depending on the stability profile of a product in its formulation buffer and its storage duration. An increasing need to decouple drug-substance and drug-product manufacturing operations makes for extended hold times. Drug-substance batch volumes can vary quite significantly from product to product — from a few hundred milliliters to several hundred liters.
A number of technologies enable bulk-scale freezing and thawing in single-use containers (28). For smaller volumes (usually ≤2 L each), square polycarbonate or PETG bottles (Nalgene) can be frozen in upright freezers that go down to −70°C. For larger volumes (2–20 L), the larger lateral dimension of bulk bottles makes for slower freezing, which increases the risk of cryoconcentration effects on protein solutions. For such volumes, a number of bag systems are now available for freeze–thaw cycles. Another advantage of bag systems comes from reduced handling when sterile-filtered drug substance is directly collected into multiple bags through a fill manifold in a closed operation.
With 6-L or 12-L volumes, Celsius FFT ready-to-use bags come in protective “clamshells.” They can be frozen in conventional −70 °C freezers (for lower throughout) or blast freezers that use liquid nitrogen (with improved heat transfer for handling larger numbers of bags). Sartorius Stedim also offers Celsius Pak bags ranging from 30 mL to 16.6 L in volume for use with Celsius freezing and thawing equipment. It is designed to eliminate the need for a “freezer farm” or blast freezer while reducing time for both freeze and thaw operations.
Process Integration
Added supply chain considerations come from the lead times associated with SU items, especially when customization is needed. This needs to be an integral part of technology transfers from process development into manufacturing operations. Certain disposable components can be considered long-lead items, with durations between order and delivery comparable to those for chromatographic resins and cell culture media. So a detailed facility fit analysis should be a key part of tech transfer.
Physical Integration: A large number and range of sterile connections are made when integrating SU equipment to create a seamless and closed manufacturing process (29). In stainless steel facilities, many transfer lines and connections are hard-piped; in facilities based on SU technology, aseptic connections for transfer and sampling need to be assembled and autoclaved before use. Low–flow-rate applications often use LuerLok fittings (from Value Plastics) with “male” and “female” parts connected by threading. Other kinds of fittings include quick-connectors with click connections (from Colder Products) and sanitary tri-clamps that use a clamp connecting two flat parts with a gasket between them. Tubing comes from a number of different vendors, including Cole Parmer (C-Flex), Saint Gobain (PharmaPure brand and SaniPure 60 heat-sealable tubing), and AdvantaPure (Advantaflex heat sealable tubing).
Aseptic connectors from a number of companies form sterile connections in a nonsterile environment. Examples include Kleenpak sterile connectors (from Pall), ReadyMate DAC (GE Healthcare), Opta SFT (Sartorius Stedim), Lynx ST (EMD Millipore), and Pure-Fit SC (Holland Applied Technologies). Tube welding also can provide a sterile connection between separate tubes without using connectors. Tube welders are available commercially from companies such as GE Healthcare (Sterile Tube Fuser), Sartorius Stedim (BioWelder), and Terumo.
SU Bioreactor Scalability: A key concern often voiced when companies consider SU bioreactor systems is whether their performance compares directly with conventional bioreactors. Transferability of operations is a greater concern for production bioreactors than for other steps in manufacturing. Below we present some data on scalability of mammalian cell culture processes in SU systems. Our data augment what has already been published (21).
Characterization: Disposable bioreactor bags contain spargers and impellers that vary in design by vendor. Most suppliers have a fixed impeller design and several options for spargers (e.g., in the number and diameter of holes). Mixing conditions in a given disposable bioreactor need to be fully characterized. Sparging rate, agitation rate, and working volumes are key factors that affect mixing characteristics.
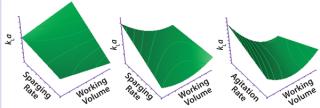
Figure 2: ()
The gas mass-transfer coefficient (kLa) for oxygen is the most important response to characterize (Figure 2). A system with low kLa will require high volumetric oxygen flow to attain sufficient levels of dissolved oxygen. A gassing-out method is used with a salt solution contained in a disposable bioreactor bag to determine kLa under different conditions. Mixing time is another response that needs to be evaluated. It is determined by adding acid or base to a bioreactor and recording the elapsed time before pH values equilibrate.
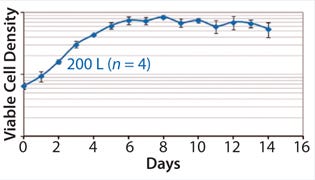
Figure 3:
Although it’s important to maintain a sufficiently high kLa and low mixing time, the scaling factor that sh
ould be used for transferring processes from one type of bioreactor to another is power input per unit volume (P0/v). The ungassed power consumption, P0, is determined using the following equation:
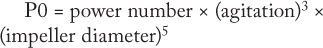
That power number depends on impeller geometry. Some disposable bioreactors have unique impeller designs. Their power numbers are very different from the usual marine-type impellers used in stainless-steel bioreactors. And it is important that the accurate power number be used when P0/v is calculated.

Figure 4: ()
In some cell culture operations, tip speed (normalized agitation rate, π × diameter × revolutions per minute) is used as a scaling factor because of concern about shear damage to cells at high tip speeds. Matching that speed across scales often yields low agitation rates in large-scale reactors. That in turn leads to insufficient kLa and P0/v values, which negatively affect results. Our recommended platform scale-up/scale-down approach for disposable bioreactors is to use P0/v and maintain normalized gas-flow rates (VVM, volumetric gas flow/liquid volume) across scales.
Technology Transfer: With projects that have been run at scale previously, P0/v is the primary criterion for transferring a process from an existing bioreactor type to a disposable bioreactor platform. We advise matching the sparging strategy as much as possible. Possible sparging strategies involve choosing between micro and macro spargers and between dual and single spargers. The preferred gassing strategy is to use dual microspargers for efficient oxygen supply and a multiple-hole wand to supply large air bubbles for efficient removal of dissolved CO2. We have used that kind of set-up extensively at KBI Biopharma for multiple programs. The data below indicate success in achieving reproducible high performance.
Figures 345 show average viable-cell density, viability, and product concentration from a 200-L Xcellerex disposable bioreactor. The y-error bars represent variability (standard deviation) across four runs, which were performed sequentially with separate vial thaws and seed trains for each. Culture performance was remarkably consistent and matched historical data from large-scale stainless-steel bioreactors and a different type of disposable unit. These data demonstrate success of the aeration strategy described above.
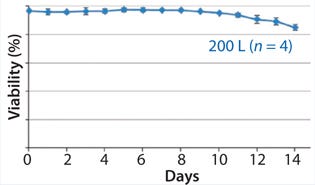
Figure 4:
From Glass to Disposable Bioreactors: KBI’s process development platform includes shake-flask evaluation followed by 3-L glass bioreactor runs. Chosen conditions then run in 15-L glass bioreactors to demonstrate scalability and supply material for downstream and analytical process development. The final process operates in 200-L and 2,000-L disposable bioreactors (from Xcellerex). Scale-up is again based on P0/V and similar VVMs maintained across scale.
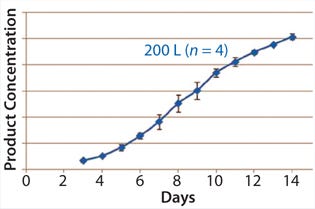
Figure 5:
Figures 678 are cell culture profiles obtained across scales for a second program. Viable-cell densities, viabilities, and product concentrations matched well across the 3-L, 15-L, 200-L, and 2,000-L scales. These results demonstrate seamless scale-up achieved across scales using a disposable bioreactor platform once aeration and agitation strategies are properly understood.
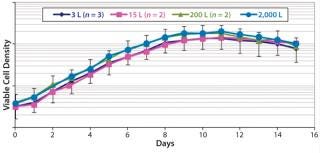
Figure 6: ()
Scale-UpIs Key
With the increasing interest in SU facilities, these technologies are rapidly evolving and expanding in scale. Effective integration is essential for seamless execution of an end-to-end single-use process in biomanufacturing.
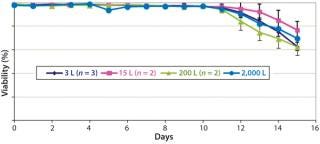
Figure 7: ()
Cell culture data across a range of scales — from laboratory to pilot plant and manufacturing — spanning fixed and single-use systems indicates how seamless process performance can be achieved across those scales with SU bioreactors. Our results are qualitatively similar to those expected with stainless-steel bioreactors. Both process reproducibility and scale-up are demonstrated for the disposable platform.
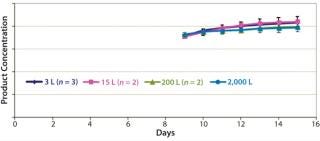
Figure 8: ()
About the Author
Author Details
Corresponding author Abhinav A. Shukla is vice president of process development, Sigma Mostafa is director of upstream process development, Mark Wilson is director of downstream process development, and Dirk Lange is vice president of manufacturing operations at KBI Biopharma, 1101 Hamlin Road, Durham NC 27704; 1-919-479-9898; [email protected], www.kbibiopharma.com.
1.) Zheng, R. 2010. The Game Changer: Transforming the Biopharmaceutical Landscape Through Single-Use Technologies. BioProcess Int. 8:S4-S9.
2.) Levine, H. 2010.The Use of Disposable Technology for Downstream ProcessingCHI Immunotherapeutics and Vaccine Summit, Cambridge:16-19.
3.) Rao, G. 2009. Disposable Bioprocessing: The Future Has Arrived. Biotechnol. Bioeng. 102:348-356.
4.) Whitford, W. 2010. Single-Use Systems As Principal Components in Bioproduction. BioProcess Int. 8:34-44.
5.) Pora, H, and B. Rawlings. 2009. A User’s Checklist for Introducing Single-Use Components into Process Systems. BioProcess Int. 7:9-16.
6.) Williamson, C, R Fitzgerald, and A. Shukla. 2009. Strategies for Implementation of a BPC in Commercial Biologics Manufacturing. BioProcess Int. 7:24-33.
7.) Wilson, J. 2006. A Fully Disposable Monoclonal Antibody Manufacturing Train. BioProcess Int. 4:S34-S36.
8.) Fuller, M, and H. Pora. 2008. Introducing Disposable Systems into Biomanufacturing: A CMO Case Study. BioProcess Int. 8:30-36.
9.) Whitford, W. 2010. Using Disposables in Cell-Culture Based Vaccine Production. BioProcess Int. 8:S20-S27.
10.) Eibl, D, T Peuker, and R. Eibl Eibl, R and D. 2011.Single-Use Equipment in Biopharmaceutical Manufacture: A Brief IntroductionSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley and Sons, Hoboken:1-11.
11.) Singh, V. 1999. Disposable Bioreactor for Cell Culture Using Wave Induced Agitation. Cytotechnol. 30:149-158.
12.) Haldankar, R. 2006. Serum-Free Suspension Large-Scale Transient Transfection of CHO Cells in WAVE Bioreactors. Mol. Biotechnol. 34:191-199.
13.) Eibl, R, C Loffelholz, and D. Eibl Eibl, R and D.. 2011.Single-Use Bioreactors: An OverviewSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley and Sons, Hoboken:33-51.
14.) Eibl, R, and D. Eibl. 2009. Application of Disposable Bag Bioreactors in Tissue Engineering and for the Production of Therapeutic Agents. Adv. Biochem. Eng./Biotechnol. 112:183-207.
15.) Tappe, A, and U. Gottschalk Eibl, R and D. 2011.Single-Use Downstream EquipmentSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley and Sons, Hoboken:91-103.
16.) Laukel, M. 2011. Disposable Downstream Processing for Clinical Manufacturing. BioProcess Int. 9:S14-S21.
17.) Pais-Chanfrau, J, K Zorrilla, and E. Chico. 2009. The Impact of Disposables on Project Economics in a New Antibody Plant: A Case Study. BioPharm Int. 22:12.
18.) Farid, S. 2005. Decision Support Tool for Assessing Biopharmaceutical Strategies Under Uncertainty: Stainless Steel vs. Disposable Equipment for Clinical Trial Material Preparation. Biotechnol. Progr. 21:486-497.
19.) Sinclair, A. 2008. The Environmental Impact of Disposable Technologies. BioPharm Int..
20.) Colton, R. 2007. Recommendations for Extractables and Leachables Testing, Part 1: Introduction, Regulatory Issues, and Risk Assessment. BioProcess Int. 5:36-44.
21.) Smelko, J. 2011. Performance of High Intensity Mammalian Fed Batch Cell Cultures in Disposable Bioreactors. Biotechnol. Progr. 27:1358-1364.
22.) Shukla, A, and J. Kandula. 2008. Harvest and Recovery of Monoclonal Antibodies from Large-Scale Mammalian Cell Culture. BioPharm 21:34-45.
23.) Yigzaw, Y. 2006. Exploitation of the Adsorptive Properties of Depth Filters for Host-Cell Protein Removal During Monoclonal Antibody Purification. Biotechnol. Progr. 22:288-296.
24.) Kandula, S. 2009. Design of a Filter Train for Precipitate Removal in Monoclonal Antibody Downstream Processing. Biotechnol. Applied Biochem. 54:149-155.
25.) Deakin, L. 2011. Scalable, Disposable Filtration Systems Address Market Challenges. Filtration+Separation 48:20-22.
26.) Pegel, A. 2011. Evaluating Disposable Depth Filtration Platforms for MAb Harvest Clarification. BioProcess Int. 9:52-55.
27.) Strahlendorf, K, and K. Harper. 2010. Mixing in Small-Scale Single-Use Systems. BioProcess Int. 8:42-49.
28.) Riesen, N, and R. Eibl Eibl, R and D. 2011.Single-Use Bag Systems for Storage, Transportation, Freezing, and ThawingSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley and Sons, Hoboken:13-20.
29.) Boehm, J. 2010. Single-Use Connections Enable Advancements in Single-Use Aseptic Processing. BioProcess Int. 8:S32-S35.
30.) Gottschalk, U. 2009. Disposables in Downstream Processing. Adv. Biochem. Eng./Biotechnol. 115:171-183.
31.) Ullah, M. 2010. Optimizing Upstream Processes to Single-Use. BioProcess Int. 8:S28-S31.
You May Also Like