Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
 It is widely accepted that standardization of single-use designs and assemblies would be beneficial to the biopharmaceutical industry, providing it quickly with simple and economical solutions. Meanwhile, as implementation of single-use technology increases across the biopharmaceutical industry, suppliers are struggling to keep up with demand. That has been evident particularly in current supply issues caused by the COVID-19 pandemic. A widely adopted single-use standardization approach could help alleviate such supply issues. That would not only benefit the industry by helping to ensure timely production, but it also would help patients by increasing their access to the therapies they need.
It is widely accepted that standardization of single-use designs and assemblies would be beneficial to the biopharmaceutical industry, providing it quickly with simple and economical solutions. Meanwhile, as implementation of single-use technology increases across the biopharmaceutical industry, suppliers are struggling to keep up with demand. That has been evident particularly in current supply issues caused by the COVID-19 pandemic. A widely adopted single-use standardization approach could help alleviate such supply issues. That would not only benefit the industry by helping to ensure timely production, but it also would help patients by increasing their access to the therapies they need.
Industry groups such as BioPhorum, ASTM International, and some individual suppliers have made attempts to influence widespread adoption of single-use standard designs, but most of those programs have been unsuccessful in achieving that goal. The situation can be attributed to a number of barriers preventing substantial adoption. For one thing, it’s too difficult and complex an undertaking to change validated production assemblies, which requires significant resources. Assembly design and procurement procedures are inconsistent within company networks. Single-use suppliers are reluctant to adopt industry-wide standardization efforts; they typically want to provide their own, differentiated components. Meanwhile, end users usually promote their own chosen platforms rather than taking a holistic, industry-wide approach. Where standardization has been adopted, it often has come as a result of an end user’s own site- and/or corporate-level in-house initiative.
The lack of single-use standardization has resulted in a proliferation of similar designs by many suppliers. That has led to supply chain complexity, large inventories, and high numbers of stock keeping units (SKUs) requiring significant resources for successful management. The single-use industry needs fewer and high-quality standard SKUs that can be provided by multiple suppliers. In effect, it needs a bold, long-term vision driven by end-user companies to realize the benefits of standardization.
A Standard Approach and Single-Use Design Tool: Recognizing the significant potential of single-use standardization and the lack of progress in facilitating its wider adoption, PM Group began its own single-use standardization initiative five years ago. This initiative began with workshops involving key biomanufacturing companies and SU suppliers.
Those led to development of our Standard Disposable Design (SDD) approach. It focuses on the dimensional standardization of single-use assemblies used in media and buffer applications and incorporates up to 350 predesigned assemblies ranging from basic filter sets, tubing manifolds, and three-dimensional (3D) bags all the way up to 1,000-L systems. In essence, the SDD concept covers the most straightforward and common single-use assemblies. It does not include complex single-use equipment such as bioreactors, chromatography systems, or tangential-flow filtration (TFF) assemblies. Five major single-use suppliers — Artesyn, Aseptic Group, Avantor, ESI Technologies Group, and Sartorius — have agreed to be part of this initiative and help move the industry forward toward supplying SDD-standard parts.
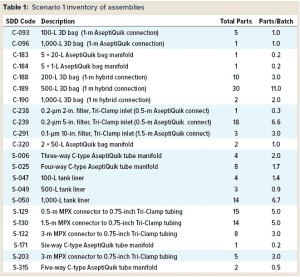
The SDD approach differs from other standardization initiatives. It was developed by involving a broad cross section of the biopharmaceutical industry ecosystem, from end users and suppliers to engineering companies. As a result, it already is more developed than other projects and has been used by different end users at a number of production sites. Furthermore, PM Group has developed a design tool to assist in SDD adoption by identifying and diagramming single-use components required in biomanufacturing processes.
Our Intention: Here we introduce our innovative approach to single-use standardization and identify the benefits that it can bring to both end users and single-use suppliers. With a standard monoclonal antibody (MAb) process as a case study, we compare scenarios using SDD and custom assemblies. Our analysis considers the number and cost of assemblies as well as inventory storage requirements. We also describe our design tool and highlight its usefulness. The approach presented herein could bring savings of over a million euros, combining direct savings on single-use assemblies and indirect reduction of tied-up capital in end-user warehouses.
Making a Business Case
For End Users: Single-use design standardization provides many benefits to end users:
shortened inventory hold times that optimize limited warehouse capacities
reduced lead times with provision of standard-dimension parts from multiple single-use suppliers
increased design speed (using the single-use design tool) with predominant use of standard parts
simplicity of ordering, with each part identified by a unique number.
The case-study results described below identify potentially significant cost savings for a typical MAb process over the course of a year when SDD-standard assemblies are used for media and buffer preparation. That conclusion is based on current SDD pricing.
Although SDD adoption is relatively limited, it is growing. With increased adoption involving many single-use suppliers, the resulting mass production of standardized bags, manifolds, and filter sets would place downward pressure on costs, both for suppliers and end users.
For Single-Use Suppliers: Past reluctance of single-use supplier companies to engage fully in industry-wide standardization initiatives has been a major obstacle to achieving large-scale adoption. The general position is understandable based on reimbursement models and how suppliers secure long-term customers through development of custom assemblies. Those can tie customers effectively to particular single-use suppliers for the duration of a given product’s life cycle. Custom assemblies designed specifically for single customers might account for up to 70% of SU-supplier SKUs.
We believe that widespread adoption of single-use standardization will succeed only if it is driven by the end-user community. Yet suppliers play a key role in making it happen. A number of them have recognized the potential benefits that standardization can bring. Sartorius, a major global supplier, has highlighted the following:
improvement in lean manufacturing
elimination of design and qualification costs
reduction in parts and finished-product inventories
streamlined documentation
increased possibilities for automation
shortened delivery times
improvements in competitive pricing
facilitation of subsupplier management
creation of new opportunities through the SDD initiative.
Reducing single-use complexity for both users and suppliers provides a means to improve quality and robustness, ensure availabilities, and make the overall supply chain more cost effective. Standardization will not prevent differentiation among single-use suppliers, which need not rely on basic functional design. Differentiation comes from a number of factors all linked to implementation at end-user sites. The real value that single-use suppliers add is not determined by what they do, but rather by how they do it. That is defined by a combination of single-use product and nonproduct attributes. Those can include specific differentiating technologies such as filter membranes, specific material-quality attributes such as robustness or cell-growth characteristics, documented qualification packages, supply assurances, single-use system integrity (SUSI) strategies, technical support capabilities, quality control systems, delivery reliability and abilities, and other competencies.
Therefore, single-use suppliers need not fear dimensional standardization. Such standardization of design does not equate to commoditization of single-use products, and opportunities abound to impart differentiation among single-use suppliers while obtaining the commercial and operational benefits highlighted above.

Figure 1: Monoclonal antibody manufacturing process
Case Study: Basic MAb Process
To demonstrate the business case and benefits of SDD adoption, we selected single-use components for a standard fed-batch MAb process from the SDD database library of predesigned assemblies. This process is well defined, resides in the public domain, and predominantly operates using single-use technology. Figure 1 lists the different unit operations. Part of our buffer and media strategy was to consider that advanced media and buffer preparation would be possible for multiple batches. To remove additional complexities, only assemblies directly used in buffer/media preparations and holding were included in our analysis. The case study covers two scenarios using standardized and custom assemblies (Figure 2).
In Scenario 1 (Standard Assemblies), all single-use assemblies used in the buffer and media operations came from the SDD database library. This includes tank liners for solution preparations, filters, tubing manifolds, and hold bags for media and buffers.
Scenario 2 (Custom Assemblies) takes a more traditional approach to the single-use assemblies. As such, it assumes that media and buffer sterilizing filters are supplied with hold bags as single assemblies.
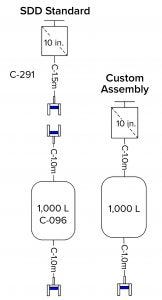
Figure 2: Example SDD standard bag–filter assembly compared with a custom bag and attached filter
The advantage of such an arrangement is that it requires one less connection (between the filter and the bag). However, that approach incurs several disadvantages. Dedicating one filter to one bag is an expensive option because it prevents that filter from being used for other bags. The number of bags connected is set (one to one). That could lead to wasting unused bags or not having enough bags attached, thus requiring creation of yet another custom bag assembly. If the same bag can be attached to different types of filters, the total number of assemblies increases, and a filter change would generate a separate SKU.
For transportation from a supplier to a user’s manufacturing facility, single-use filters need to be packed such that they do not damage attached bags. Especially for manifold assemblies with filters connected to multiple bags, the ergonomics are difficult in packaging assemblies and in handling the resulting “octopus” of tubing upon receipt and opening. Filters often are process specific, so discontinuing a process would include discarding and wasting unnecessarily any remaining sets of filters and hold bags.
 The Single-Use Design Tool
The Single-Use Design Tool
To select single-use components required for the two scenarios described above, we used PM Group’s Single-Use Design Tool application. It was created to fill a gap between supplier drawings and standard process and instrument drawings (P&IDs). Using the tool in this case study helps illustrate the two scenarios and to calculate the number of assemblies in each one. Supplier drawings are created primarily for a supplier’s manufacturing department to separate assembly drawings from bills of materials. Formatting of supplier drawings varies slightly among vendors, which makes it difficult for end users to understand how — and whether — assemblies fit together. Supplier drawings typically do not provide information about how many assemblies will be required for each batch.
The P&ID format has been perfected over many years to serve as a master document for visually identifying equipment, piping, instrumentation, and controls for process facilities based on stainless steel equipment. However, it does not adapt well to single-use process facilities. For example, if bag size, tubing length, outer diameter, inner diameter, connector type, and tubing composition are incorporated into a P&ID, then the information becomes too complex to convey clearly.
 The design tool concept was created to give a P&ID-type overview for single-use systems. It does so by concentrating only on bag size, tubing length, outer diameter, inner diameter, connector type, and tubing composition. This approach provides a number of benefits and functionalities (1). It is simple and thus fast to use. It facilitates technological transfer by quickly creating an “as-built” drawing that is particularly important during transfer operations. The tool facilitates process visualization, allowing users to see their whole facility on a single drawing. That helps with open-closure risk analysis by making it easy to identify specific connections. The tool creates an inventory of custom and standard assemblies and provides a strong platform for training. It depicts custom parts and standard assemblies to build a visual representation of the whole process quickly. It develops single-use inventory and converts supplier drawings into simple drag-and-drop icons in a Microsoft Visio interface. The tool thus allows for rapid creation of “kitting” drawings by copying and pasting from an original drawing onto additional sheets, ensuring that nothing is missed.
The design tool concept was created to give a P&ID-type overview for single-use systems. It does so by concentrating only on bag size, tubing length, outer diameter, inner diameter, connector type, and tubing composition. This approach provides a number of benefits and functionalities (1). It is simple and thus fast to use. It facilitates technological transfer by quickly creating an “as-built” drawing that is particularly important during transfer operations. The tool facilitates process visualization, allowing users to see their whole facility on a single drawing. That helps with open-closure risk analysis by making it easy to identify specific connections. The tool creates an inventory of custom and standard assemblies and provides a strong platform for training. It depicts custom parts and standard assemblies to build a visual representation of the whole process quickly. It develops single-use inventory and converts supplier drawings into simple drag-and-drop icons in a Microsoft Visio interface. The tool thus allows for rapid creation of “kitting” drawings by copying and pasting from an original drawing onto additional sheets, ensuring that nothing is missed.
Once an as-built drawing is created, it can be studied to enable potential simplifications, reductions in single-use cost, and optimization of warehouse space use. An operations team can implement the drawing readily because it is relatively easy to use. Thus, operations staff can make as-built modifications and keep their single-use system drawings up to date. Engineering groups also can use this tool to manage and control single-use parts and assemblies within an organization network.
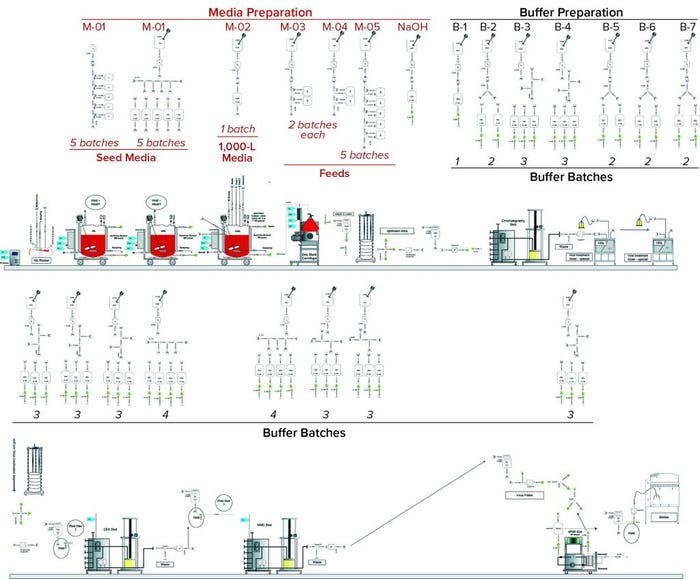
Figure 3: Scenario 1 (standardized) assemblies
Single-Use Assemblies
Standard Assemblies: Figure 3 shows the design for this MAb process using SDD and custom assemblies. The diagram shows media-preparation operations in the top row and buffer-preparation operations in the third row. Core process operations — upstream and downstream in the second and fourth rows, respectively — are not considered in our analysis. Table 1 summarizes the assembly types and numbers to be considered in this design.
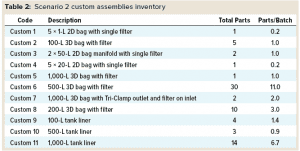
Custom Assemblies: The same MAb process design used with custom assemblies is indicated in Figure 4. It follows the same structure as that for Scenario 1, with media- and buffer-preparation operations depicted in the top and third rows, respectively. Table 2 summarizes the inventory of custom assemblies for the same design.
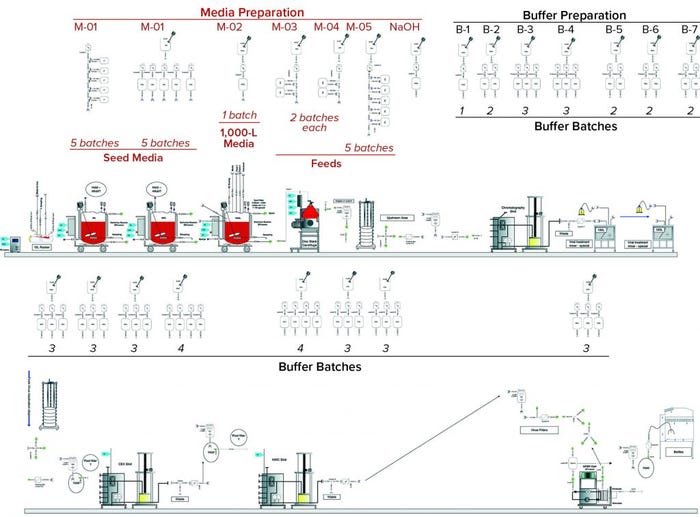
Figure 4: Scenario 2 (customized) assemblies
Comparison
Below we compare both scenarios based on inventory storage and the number and cost of assemblies for each. Table 3 compares component assembly numbers and costs. Cost data for SDD assemblies come from a Sartorius price list. The same company also specified costs for the custom designs.
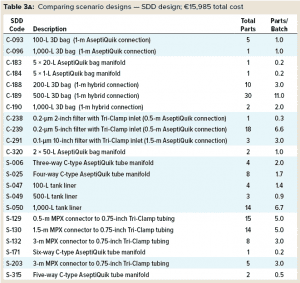 Custom Assembly Design: Table 3 shows that implementing SDD assemblies in Scenario 1 resulted in a greater number of assemblies being used than were required in Scenario 2. That’s because the SDD approach breaks down complex custom assemblies into smaller, more universal subassemblies. Doing so is a key principle of the standardization approach. The downside is a corresponding increase in the number of aseptic connectors/tubing welds required to equip a process plant. However, the improved robustness and reliance of aseptic connectors dramatically reduces the impact on an overall design.
Custom Assembly Design: Table 3 shows that implementing SDD assemblies in Scenario 1 resulted in a greater number of assemblies being used than were required in Scenario 2. That’s because the SDD approach breaks down complex custom assemblies into smaller, more universal subassemblies. Doing so is a key principle of the standardization approach. The downside is a corresponding increase in the number of aseptic connectors/tubing welds required to equip a process plant. However, the improved robustness and reliance of aseptic connectors dramatically reduces the impact on an overall design.
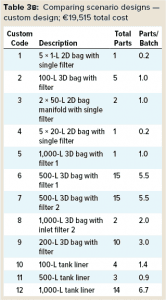 Total Assembly Costs: Scenario 1 provides a significant cost savings per batch — €3,975 per batch (€19,515 – €15,985 = €3,975) — compared with Scenario 2. So a typical single-use facility such as the one described herein making two batches per week for 46 working weeks each year would deliver a savings of €365,700/year if a SDD approach was adopted. SDD assemblies are less expensive because their designs are simplified. Note that although the overall number of assemblies was higher for Scenario 1, it still provides a cost advantage compared with Scenario 2.
Total Assembly Costs: Scenario 1 provides a significant cost savings per batch — €3,975 per batch (€19,515 – €15,985 = €3,975) — compared with Scenario 2. So a typical single-use facility such as the one described herein making two batches per week for 46 working weeks each year would deliver a savings of €365,700/year if a SDD approach was adopted. SDD assemblies are less expensive because their designs are simplified. Note that although the overall number of assemblies was higher for Scenario 1, it still provides a cost advantage compared with Scenario 2.
Inventory Storage: Typically, single-use facilities are designed to hold up to six months’ worth of parts inventory to mitigate supply-chain risk. One key advantage of standardization is that the supply chain for standard single-use parts would be much stronger than it would be otherwise, not only because they are “standard,” but also because multiple suppliers can provide the same part. So inventory stocks at a manufacturing facility can be reduced significantly without risking stoppages. With a reduction from six months of assembly inventory to two months, the value of stock on hand drops from €995,240 for Scenario 2 to €271,000 for Scenario 1, providing a €724,240 reduction in tied-up capital. That makes a significant impact on the size of the warehouse needed. Such a reduction in inventory allows for smaller warehouses in new facilities, with 40 pallet spaces required for the SU components in Scenario 1 compared with 101 pallet spaces required for Scenario 2.
Discussion
It is widely accepted that single-use standardization will benefit the biopharmaceutical industry’s end users and thus ultimately help patients, too. However, despite industry initiatives to promote standardization, wide adoption has not materialized. Some end-user companies have tried to standardize within their own manufacturing networks, but such initiatives do not fully realize the benefits that an industry-wide initiative would deliver.
In collaboration with multiple biopharmaceutical companies and single-use suppliers, PM Group developed a broader single-use standardization initiative. The key difference is that it is supported by a number of single-use suppliers. This program already is operational, with a number of biomanufacturing sites adopting the SDD approach.
Although many single-use suppliers have been reluctant to promote standardization concepts in the past, those now supporting the SDD initiative have recognized the results that it can provide, not only to themselves as a suppliers, but also to users and patients. Benefits include logistical gains, increased certainty and speed of supply, and cost savings.
In our case-study scenarios, we focused on standardization of assemblies used for support operations associated with media and buffers. We did not include complex single-use equipment such as bioreactors. Nevertheless, two major benefits became clear: an annual cost saving of €365,700 on single-use assemblies and a significant reduction in single-use inventory capital of €724,240 along with elimination of 61 pallet spaces in the supporting warehouse.
Using the single-use design tool provides additional advantages in the execution of single-use projects. It quickly creates a drawing of a whole process that identifies all single-use assemblies involved. The single-use design tool also collates an inventory of single-use assemblies so that costs can be determined quickly by SDD suppliers.
The SDD approach and single-use design tool were developed specifically to address the single-use standardization goals of the biopharmaceutical industry. They were created in collaboration with both users and suppliers, and the resulting introduction has demonstrated significant cost and time savings. Widespread adoption will bring more cost savings and increased efficiencies in the future.
Acknowledgment
We acknowledge the efforts of Dave Wolton (engineering technology lead at Takeda) and his support for the SDD initiative, from our initial ideations through development of the 350 designs currently available. He remains a driving force for standardization within the biopharmaceutical industry.
Reference
1 Standard Disposables Design (SDD™). PM Group: Dublin, Ireland, 2021; https://www.pmgroup-global.com/what-we-do/sectors/pharmaceuticals/standard-disposables-design.
Corresponding author Javier Lozano is UK head of process engineering, and Austin Lock is group head of technology at PM Group, Trinity Park, Bickenhill Lane, Birmingham, B37 7ES, UK; 44-121-767-6700; [email protected]; [email protected].
Standard Disposable Design, SDD, and Single-Use Design Tool are registered trademarks of PM Group. AseptiQuik and MTX are registered trademarks of Colder Products Company.
You May Also Like