Bosch Packaging Technology has been supplying the pharmaceutical industry with final fill solutions for over 30 years. During that time, Bosch has continued to focus on quality and industry-leading customer-focused solutions that provide the best return on investment over time.
Filling Systems
Bosch is the premier supplier of final fill–finish solutions to the pharmaceutical industry.
As a single-source supplier with the industry’s widest portfolio of solutions, Bosch can supply the type and scale of equipment you require. We offer systems for bulk syringes, vials, ampules, cartridges, and prefilled syringes from laboratory scale to high-speed production. Our project management team will work with you as your system is pretested in our facility to ensure that it performs as promised before delivery.
Sterilization Systems
With the acquisition of SBM, Bosch now offers a full line of sterilizations systems.
Specially made for the rigors of the pharmaceutical industry, Bosch sterilizers are designed for max...
Expertise
Cobra Biologics is a rapidly expanding international contract manufacturing organization (CMO) of biologics and pharmaceuticals for clinical and commercial supply. Cobra has three good manufacturing practice (GMP)-approved facilities, each with expertise tailored to serving our customers across the world. We offer a broad range of integrated and stand-alone contract services, stretching from cell line and process development through to fill and finish for the supply of investigational medicinal products and commercial production.
Experience
For over 16 years, we have taken pride in being a trusted manufacturing provider, delivering what we promise and helping our customers to develop medicines for the benefit of patients. Cobra provides manufacturing technologies, platforms, and solutions to the biopharmaceutical industry covering antibodies, recombinant proteins, viruses, phage, DNA, whole-cell vaccines and therapeutics as well as biologics and small-molecule active pharmaceutical ingredient (A...
The commercial prefilled syringe line at Cook Pharmica can process up to 600 syringes per minute, 36,000 syringes per hour, or 70 million syringes per year. The automated, high-speed syringe line is fully enclosed in a barrier isolator to provide the highest level of aseptic processing available by removing the human element from the entire process. With the ability to operate in smaller batches or full-scale campaigning, the prefilled syringe line is capable of bridging clinical programs into commercial production.
The process starts in a separate tub-loading room where tubs are loaded onto a pass-
through conveyor and sent into the isolator for automatic debagging. After the tub is removed from the bag, it passes through a tunnel utilizing E-beam technology for tub decontamination. When it exits the E-beam tunnel, robotic arms remove the lid and liner to prepare the syringes for denesting and filling.
The line is capable of time-pressure or peristaltic filling, with temperature-compensating recirculatio...
The ability to successfully produce cell-based products is partially dependent on the technologies and instruments available. Volume manipulation, supernatant or culture media exchange, and cell washing of the cell product can present a significant challenge, especially when cell recovery needs to be maximized without compromising viability.
FIGURE 1: SEM images of the track-etched polycarbonate membrane used within LOVO’s proprietary spinning membrane separation device; view normal to the membrane surface (left), cross-sectional view of the membrane (middle), and close-up of the 4-μm pores (right)
LOVO is a multiuse, bench-top instrument that pumps a cell solution through a spinning membrane filtration device to allow for rapid fluid management and fast, efficient cell processing. At its core, LOVO leverages Fresenius Kabi’s proprietary spinning membrane separation device, which uses a 4-μm track-etched polycarbonate membrane (Figures 1 and 2), and allows for separation of target cells through size disc...
Fujifilm Diosynth Biotechnologies (FDB) leverages over 15 years of experience in bioprocess development and manufacturing to successfully deliver robust process designs to its clients. FDB has executed a series of innovation programs to enable rapid development of cell culture processes, including the coming launch of a new Chinese hamster ovary (CHO) expression system. This application note describes how outputs from media screening and upstream process development innovation programs were applied to provide significant productivity and process robustness improvements to an early stage client program.
Results
Toolbox Media/Feed Screening: FDB developed a toolbox of high-performance basal media and feeds by screening numerous commercial and prototype media with diverse CHO cell lines.
FIGURE 1: Cell growth trends with various basal and feed media
combinations
In this case study, 10 basal media and feed media combinations were selected for fed-batch production trials in shake flasks. Varying degrees of cel...
The Xuri cell expansion systems (Xuri W25 and W5) provide a functionally closed, single-use bioreactor that requires fewer operator intervention steps, reduces the risk of contamination, and controls the cellular environment to maximize cell yield. Data presented here highlight that at a cellular level, results from different bioreactors may appear similar, but differences can exist at the receptor expression level.
Expansion of T Cells
FIGURE 2: An example of how the pO2 saturation can vary during the
expansion of T cells; the lack of effective mixing in the STR bioreactor
reduces the capability of the stirred-tank system to maintain an
acceptable level of dissolved oxygen.
FIGURE 1: An example of how pH can vary during the expansion of T
cells; at higher cell densities, the stirred-tank bioreactors, which lack
perfusion features, are unable to maintain a suitable pH, resulting in the
operator having to exchange medium to correct the pH.
Peripheral blood mononuclear cells (PBMCs) were expanded in static ...
NNE Pharmaplan has developed a new facility concept called Bio on demand™, which enables facilities to be established in one to two years. The result is a flexible facility that is fully operational, locally compliant, and contains functioning quality systems. Bio on demand™ involves a high degree of single-use technology to ensure cost-effective production and fast establishment.
Facilities based on our Bio on demand™ concept can be delivered globally, but they are primarily intended for growth markets where time-to-market is particularly essential.
Full Package for Full Predictability
The Bio on demand™ concept includes engineering and supply of a facility as well as related quality systems, standard operating procedures, and coordination of necessary quality tests.
When executing a project, NNE Pharmaplan’s overall objective is that the customer develops new capabilities and grows existing ones. And by not only providing a facility but also a full turn-key solution, we allow our customers to focus on c...
Unlike typical pharmaceuticals, cell therapies rely heavily on the manufacturing process for product attributes. As more products move out of the clinic and toward commercialization, the manufacturers of cell therapies are realizing that commercialization is unlikely to be successful without an effective methodology for manufacturing development.
At PCT, we recognize that a successful commercial manufacturing vision includes the ability to provide consistently high product quality at a reasonable cost of goods that meets demand over the commercial life of a product. To help our clients realize this vision, PCT is proud to offer its innovative scientific and engineering expertise to plan and execute development by design (DbD) methodology.
PCT’s industry-leading DbD approach applies a series of principles to our clients’ cell therapy manufacturing processes to help guide strategy and provide structure and discipline to plan for and address commercialization risks before it is too late to efficiently addres...
Pyramid Laboratories, Inc. is a contract aseptic manufacturing and analytical services organization for sterile injectable drugs. Pyramid provides expertise in formulation and process development, aseptic filling for vials and syringes, and lyophilization applications. Pyramid has established a reputation of exceptional performance, integrity,
and quality.
Technical Services
Pyramid offers a wide array of services for all phases of drug development and production including
Facilities
Pyramid Laboratories, Inc. is located in southern California in the United States. Our facilities are housed in three buildings covering more than 70,000 ft
2
. The combination of Pyramid’s manufacturing facilities and state-of the-art laboratory allow the company to offer the pharmaceutical and biotech industry both analytical and manufacturing support capabilities. An intensive in-house quality assurance and quality control program is maintained to ensure that clients receive high-quality products. Pyramid expanded its por...
Identifying the most appropriate contract manufacturer that is most likely the best match for your lead pharmaceutical candidate is a true challenge. Rentschler is a biopharmaceutical manufacturer with a proven track record for over 40 years. Focused on mammalian cell lines, our experience covers the development, production, and aseptic filling of recombinant proteins such as cytokines, enzymes, monoclonal antibodies, and fusion proteins in compliance with international GMP standards (EMA/FDA). We don’t just claim to be reliable and experienced; our many successful projects and long-lasting client relationships show that we are.
TurboCell™ Technology
Rentschler’s proprietary TurboCell™ platform enables the simultaneous production of up to 20 early stage drug candidate variants in stable CHO cell lines. TurboCell™ is a technology for the fast generation of highly stable recombinant mammalian (CHO-K1) cell lines based on cassette exchange (RMCE). The cassette allows the precise integration of the gene of in...
Sandoz is one of the leading specialists in biotech development and manufacturing. Comprehensive know-how is based on more than two decades in microbial and mammalian cell culture production at commercial scale. Today Sandoz offers a broad variety of integrated services, including innovative expression systems, process development, scale-up, and production of recombinant products derived from microbial systems and mammalian cell culture in state-of-the-art facilities.
Our expertise is based on numerous projects successfully performed in collaboration with our customers. Currently, Sandoz is manufacturing more than 25 different recombinant products for well-known pharmaceutical and biotechnology companies located in the United States, Europe, and Japan. Our FDA-approved facilities fulfill worldwide quality requirements.
Services Offered
Production of Recombinant Peptides and Proteins:
150-L, 1,300-L, 3,000-L, 13,000-L, and 40,000-L fermentors are available in our facility in Kundl, Austria. Each fermentat...
Contract manufacturing of biologics is more than having superior technology — it’s having experienced people who are passionate, responsive, and committed to developing and manufacturing your biotherapeutics to improve patient care. As a fully integrated contract development and manufacturing organization (CDMO), Therapure Biomanufacturing has the scientific and technical expertise to successfully deliver at every stage of product development (preclinical, clinical, and commercial). Our 130,000-ft
2
(12,000-m
2
) current good manufacturing practice (cGMP) manufacturing facility meets FDA, EMA, and HPFB regulatory standards and includes flexible clinical and commercial production suites.
Development Services
Therapure appreciates the complexity of the development process. Our range of services is supported by a deep scientific understanding of process and analytical development. Our development services include
Cell Line:
generation of well-characterized, stable mammalian cell lines
Upstream:
developmen...
Vetter, a leading provider of aseptically prefilled drug-delivery systems, operates a state-of-the-art facility at the Illinois Science and Technology Park in suburban Chicago. The site offers a central US location with a domestic and international airline hub. It is uniquely situated in a thriving biopharmaceutical region that offers an established infrastructure and prominent research institutions.
The company’s Chicago site supports preclinical through phase 2 development projects. The 27,000-ft
2
facility’s functional areas include microbiology and chemical analysis laboratories; materials preparation; compounding; aseptic CGMP filling for vials, syringes, and cartridges; quality assurance; and seamless transfer to Vetter commercial manufacturing.
DeveIoped at the urging of Vetter’s North American clients, the Chicago site supports small-batch, early stage products. Vetter has extensive experience working with biologics, including monoclonal antibodies, peptides, interferons, and vaccines.
Supporting...
Wacker Biotech GmbH is “The Microbial CMO” — the partner of choice for the contract manufacturing of therapeutic proteins in microbial systems. The company is built on more than 20 years of experience in biopharmaceutical manufacturing. Founded in 1999 as a spin-off from the Hans- Knöll Institute in Jena, Wacker Biotech is a 100% subsidiary of Wacker Chemie AG since 2005.
Wacker’s integrated service portfolio covers molecular biology, process and analytical development, and the GMP manufacturing of biologics for clinical trials and commercial supply. We round out our offering with outstanding Escherichia coli technologies that can significantly increase bioprocess efficiency and thus reduce cost of goods.
In 2014, Wacker acquired Halle-based Scil Proteins Production GmbH. The merger has leveraged complementary skills in the production of biopharmaceuticals, thus creating a strong and highly competitive microbial CMO. The acquisition has doubled our development and manufacturing capacities and has added va...
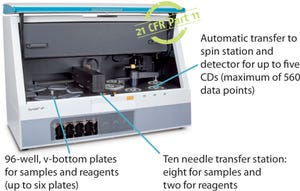
Increasing efficiency and productivity for titer and impurity bioanalytics is even more critical as a result of increasing workloads in supporting multiple programs with limited resources. Current methods such as enzyme-linked immunosorbent assay (ELISA) or high-performance liquid chromatography (HPLC) create bottlenecks in critical workflows because of throughput constraints, repeat testing, or longer assay times. The Gyrolab™ workstation is an automated immunoassay platform that can generate high-quality analytical data at high throughput using nanoliter volumes for more rapid and efficient titer and impurity immunoassays.
Gyrolab offers key advantages for applications such as protein expression, cell line development, and the detection of bioprocess impurities. It is a robust, automated platform providing high throughput, enabling data-driven decisions with minimal bench time and rapid time to results.
Gyrolab xP Workstation
Gyrolab xP workstation is an automated platform that uses proprietary microflu...
From drug and biologic development services to delivery technologies to supply solutions, we are the catalyst for your success. With over 80 years of experience, we have the deepest expertise, broadest offerings, and the most innovative technologies to help you get more molecules to market faster, enhance product performance, and provide superior, reliable manufacturing and improved results. Catalent is your strategic partner for biologic drug development success.
SMARTag™ ADC Technology for the Development of Optimized Antibody–Drug Conjugates:
Catalent has an exclusive license and comarketing agreement with Redwood Bioscience to offer customers SMARTag advanced, site-specific conjugation technology and linker–toxin combinations to develop optimized antibody–drug conjugates.
SMARTag proprietary technology can help the development of improved ADCs. It allows for precise conjugation and optimal release of the therapeutic agent, and can provide
Biomanufacturing Center of Excellence:
Our state-of-the-art f...
Environmental monitoring of clean rooms is an integral part of a pharmaceutical company’s quality control testing program. Analyzing the air, surface, and personnel within cleanrooms helps ensure the manufacturing environment meets mandated standards and that any variations can be addressed. Unfortunately, the traditional method for environmental monitoring is manual, inefficient, and time consuming. Laboratory technicians must travel, sometimes across large campuses, to collect samples in manufacturing suites. Technicians have to gown, take the samples, and travel back across the campus to the lab where samples are logged in before incubation.
After 5–7 days, the samples are removed, and each plate is manually counted. Technicians then record the counts for each plate. Paper results are keyed into a laboratory information system (LIMS). For large sites, environmental monitoring programs involve hundreds of samples being tested each day, taking time and resources that could be used for higher value activ...
Following the initial observations by Chen et al., leading manufacturers of biopharmaceuticals have determined that endotoxin (LPS) can be “masked” in biopharmaceutical formulations typically containing phosphate, polysorbate, and sugars (
1
). Being in a masked state, endotoxin is undetectable by the commonly used factor C detection methods such as the Limulus amebocyte lysate test (LAL). This phenomenon has also been denominated low endotoxin recovery (LER).
As previously shown by Reich et al. (
2
), endotoxin masking is time dependent, meaning that a defined amount of endotoxin spiked to a sample at time point zero often cannot be recovered (detected) with commonly used methods after hours or days. This is sometimes the case even within minutes of positive product controls (PPCs) initially have indicated a positive test result. Such lack of test reliability can ultimately affect patient safety.
FIGURE 1: Workflow of Hyglos sample preparation method for
unmasking endotoxin
Reich has studied the mechanis...

 +1
+1