Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
November 17, 2021
Sponsored by Frost & Sullivan
Advancements in next-generation therapies—Paving the way for novel bioprocessing solutions.
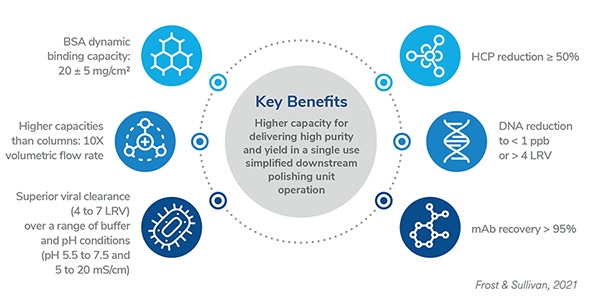
Learn about 3M Polisher ST technology which utilizes a guanidinium-functionalized polyamide membrane protected by a Q functionalized non-woven material. This Frost & Sullivan whitepaper explains how the technology demonstrates the viability of replacing the depth filtration and anion exchange chromatography (AEX) steps to achieve a simplified and cost-effective process. The platform is designed to reduce process and product related impurities and offer robust performance across a wide range of process conditions.
Biopharmaceutical products (biologics) are transforming the treatment of many diseases like cancer, rheumatoid arthritis (RA), Hepatitis C, and Multiple Sclerosis (MS). Advances in biopharmaceutical innovation have transformed the treatment paradigms, helping patients slow disease progression and even disease remission for conditions such as RA. In the US and Puerto Rico alone, biopharmaceutical companies host approximately 1,100 manufacturing plants, producing cutting edge therapies. Continued breakthroughs in biomanufacturing will be critical in addressing unmet patient needs and solving future healthcare challenges.
Monoclonal antibodies (mAbs) and recombinant therapeutic proteins are the two largest biologics segments, comprising nearly 86.0% of the total biologics market in 2019. Producing these molecules consistently at a commercial scale is both complex and costly. The complexity is demonstrated by the variety of modifications on the molecule, including post-translational modifications, such as glycosylation, and degradation products, such as oxidation and hydrolysis, which affect the efficacy and safety of the drug. Similarly, progress in the mammalian cell culture process has resulted in significantly increased product titers, but also a substantial increase in process- and product-related impurities.
Due to the diverse physicochemical properties of these impurities, there is a constant need for new technologies that offer higher productivity and improved economics without sacrificing the process robustness required to meet final drug substance specifications. This paper outlines the performance of 3M™ Polisher ST technology, which allows the downstream polishing train to be restructured and simplified and chromatographic purity standards to be met with a reduced number of chromatographic steps.
You May Also Like