Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 22, 2022
Sponsored by Savillex

Purillex bottle microbial contamination testing
The choice of materials to develop and process biopharmaceutical products has a significant influence on the quality and purity of those products. Biomanufacturers have benefited from their use of both stainless-steel and single-use materials for individual process components and entire process systems. But careful attention must be paid to material characteristics. Working with different single-use plastics, for example, means that biomanufacturers must take product-contact issues into account, including the risk of extractables and leachables.
To celebrate its 20th anniversary, BioProcess International asked industry suppliers to participate in a questionnaire. Below, I provide my insights on the important technical developments defining the biomanufacturing industry over the past two decades and what other industries can learn from those developments.
What Material Science Development Has Changed Your Work?
Use of advanced materials in bioprocessing applications has had a significant impact on my work. It is the most significant paradigm shift since the beginning of the biopharmaceutical industry’s shift from stainless-steel components to disposable plastics in the 1990s. Materials used to manufacture bioprocess single-use systems changed little since the first systems were made from polyethylene and other hydrocarbon polymers. The focus at that time was purely on material availability and basic system functionality. Based on available materials used in other industries, disposable systems were manufactured from multiple materials bound together with adhesives to meet specifications related to leak tightness, gas permeability, and rudimentary mechanical durability. Little thought was made to overall chemical “cleanliness” and compatibility of the disposable systems with processes such as freezing and shipping. It was only later that other materials were created to remedy those oversights.
An entirely new class of single-use systems was developed about 10 years ago using advanced materials called fluoropolymers. Those included partially fluorinated polymers such as polyvinylidene fluoride (PVDF) and ethylene tetrafluoroethylene (ETFE) and fully fluorinated polymers such as perfluoroalkoxy alkanes (PFA) and fluorinated ethylene propylene (FEP). Some of those materials have been used for many years in components such as filling-system tubing, stopper and syringe coatings, and analytical vials used for extractables testing. However, the real innovation was using those materials to manufacture single-use containers. Suddenly, systems could be manufactured to be truly universal, withstanding all process chemicals, temperatures, and shipping methods. That led to applying single-use storage components for strong solvents and corrosive materials and temperatures down to liquid nitrogen immersion — all with little to no extractables detected. Additional data gathered in the past few years show that single-use systems manufactured from fluoropolymers are even naturally resistant to carbon dioxide ingress during shipping on dry ice.
Another advantage of fluoropolymers is that they exhibit very low coefficients of friction. That means that they do not adhere biological materials to process surfaces, have inherent resistance to bioburden and endotoxin, and can be easily cleaned when required. A low coefficient of friction also means that liquids will drain fully from fluoropolymer-based systems because they will roll off of container surfaces.
What Can Emerging Industries Learn from Technology Advances in Bioprocessing?
Although one of the most significant technical advances in bioprocessing over the past 30 years was the introduction of disposable fluid paths and “ready-to-use” processing as part of the industry’s transition to industrialization. However, other emerging markets such as cell and gene therapies (CGTs)remain largely in R&D manufacturing mindsets. For the most part, systems used to manufacture those drug products are more compatible with clinical settings than with manufacturing facilities. The process strategy is to scale out rather than scale up, with multiple small-scale systems instead of a single large-scale train. In fact, many of those systems are largely clinical in nature and are not scalable.
At the same time, little attention has been paid to material selection and manufacturing processes for single-use systems. The focus again is on material availability and basic system functionality, with emphasis on readily available materials that will work for bioprocesses. “Can I make the product?” has been a more important question than “How should I make it to have the best outcome?” However, researchers are finding that material of construction can play a huge part in the survivability of live cells and tissues used in CGTs and regenerative medicine. Even small reductions in cell viability for a final product can mean the difference between positive and negative patient outcomes.
Spending weeks growing a patient’s tissues only to have them die during storage and shipment can be frustrating. Advanced materials such as fluoropolymers are being evaluated to help solve those types of problems. Preliminary data from live-tissue applications indicate improved viability in solid tissue samples after storage in fluoropolymer bottles as compared with those made from other materials such as polycarbonate. It is thought that the lack of leachables from fluoropolymers contributes to this phenomenon.
Other factors are the manufacture of single-use systems used for emerging industries and the controls that should be placed to ensure consistent high-quality products. For the most part, the single-use bioprocessing industry has standardized a set of materials and quality testing that ensures consistency and built-in high quality. For example, single-use systems typically are assembled in certified ISO class 7 cleanrooms that are monitored for airborne particulate contamination. Final assemblies are tested against USP <788>, which describes a method for detecting motile particles in a fluid path and wetted parts. Common materials used in research (e.g., PVC and polystyrene) are replaced by materials with low extractables. Systems are sterilized (if required) using validated processes, with accompanying shelf-life validation data to ensure that products remain sterile and fit for use for long periods.
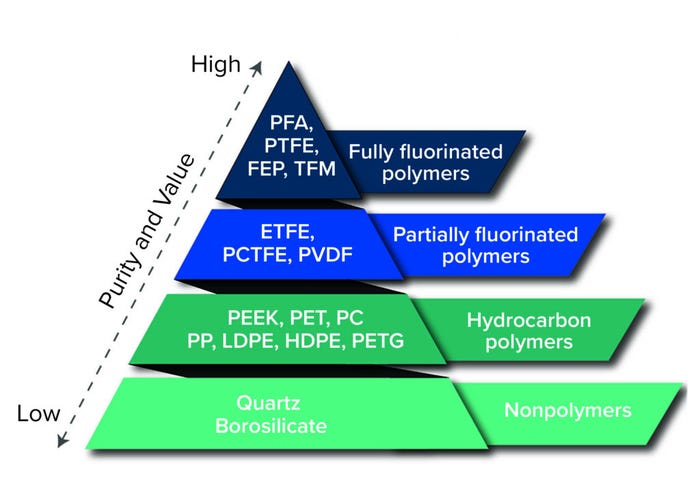
Figure 1: Materials comparison pyramid (PFA = perfluoroalkoxy alkane, PTFE = polytetrafluoroethylene, FEP = fluorinated ethylene propylene, TFM = modified PTFE, ETFE = ethylene tetrafluoroethylene, PCTFE = polychlorotrifluoroethylene, PVDF = polyvinylidene fluoride, PEEK = polyether ether ketone, PET = polyethylene terephthalate, PC = polycarbonate, PP = polypropylene, LDPE = low-density polyethylene, HDPE = highdensity polyethylene, PETG = polyethylene terephthalate glycol)
What Technical Developments Have Surprised You the Most?
The one technical development that has surprised me the most is the speed at which disposable plastic process systems have been implemented in commercial bioprocessing. I was an early adopter of disposables back in the 1990s, when the company I was with (a major biopharmaceutical manufacturer) decided to implement single-use bags for storage and dispensing of process buffers. Our laboratory was the first to test the long-term stability and sterility of those buffers when they were stored in bags. We later applied what we had learned to commercial manufacturing and implemented single-use bags during the manufacture of commercial product lots. I remember that there were no standard containers available for holding the bags, and few options for properly handling them during use. We even went as far as visiting our local discount store to buy plastic totes that could offer some level of protection for the bags.
Plastic and glass bottles and carboys also were used extensively in our facility. Many of our processes were developed for rigid containers, and most of those processes stayed in that form when commercialized. Even our bulk drug substance was filled into individual bottles for shipment to our filling sites. However, we never considered those bottles to be single-use in the same way we did bags. The reason was that presterilized bottles were a catalog item that most of us used in school and research laboratories. Every activity back then was performed manually. Fluids were transferred using pipettes, a filling bell, or by pouring. Ported bottles with dip tubes, inlet–outlet tubing, and connectors were not commercially available. We sometimes had to make our own ported bottles by drilling bottle caps and gluing in connectors.
A lot has changed in the past 20 years. However, I have learned that the movement from stainless steel to disposables does not necessarily include bags. Ported bottles and carboys are just as common as ported bags. In fact, many users are moving away from flexible bags and back to rigid plastic containers for critical process fluids because of the containers’ improved durability, ease of handling and shipping, and reduced risk of product loss.
Eric Isberg is vice president of life sciences at Savillex,
10321 West 70th Street, Eden Prairie, MN 55344; [email protected].
You May Also Like