Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
June 10, 2020
Sponsored by Cygnus Technologies
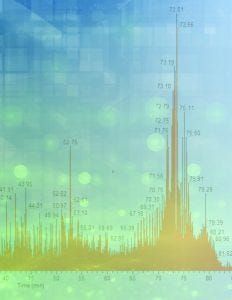 Enzyme-linked immunosorbent assays (ELISAs) remain the industry standard for process monitoring and lot-release testing for host-cell proteins (HCPs), but researchers need orthogonal tests to confirm that an ELISA is fit for purpose. On 22 April 2020, Eric Bishop (vice president of research and development at Cygnus Technologies) delivered an “Ask the Expert” presentation about his company’s solution: mass spectrometry (MS) preceded by a novel Antibody Affinity Extraction (AAE) technique. Bishop explained that an AAE step can magnify a sample’s HCP content and that such enrichment can hone subsequent MS.
Enzyme-linked immunosorbent assays (ELISAs) remain the industry standard for process monitoring and lot-release testing for host-cell proteins (HCPs), but researchers need orthogonal tests to confirm that an ELISA is fit for purpose. On 22 April 2020, Eric Bishop (vice president of research and development at Cygnus Technologies) delivered an “Ask the Expert” presentation about his company’s solution: mass spectrometry (MS) preceded by a novel Antibody Affinity Extraction (AAE) technique. Bishop explained that an AAE step can magnify a sample’s HCP content and that such enrichment can hone subsequent MS.
Bishop’s Presentation
Currently, ELISAs are the only available method for measuring ng/mL HCP levels in mg/mL levels of target protein. Unfortunately, such testing does not reveal what HCPs the assay reacted to in sampled drug substance. Thus, orthogonal evaluation is necessary. Historically, researchers relied on two-dimensional (2D) Western-blot analysis for support, but that approach inconsistently predicts how antibodies might perform in an HCP ELISA. MS can overcome such limitations, yet the sheer number of product proteins in purified drug substance overwhelms MS detectors, and that problem can cast doubt upon MS robustness.
Cygnus devised Antibody Affinity Extraction technology to enrich sample HCP content before MS analysis. An AAE antibody-coverage step begins with coupling HCP antibody to a solid support, followed by extensive conditioning and washing. Then, a sample is loaded, washed until it achieves a background signal, and eluted. The resulting eluate represents what HCPs the antibodies removed.
This process effectively amplifies what impurities reside in researchers’ sample. For instance, in-house studies of the AAE technology report 20-fold enrichment of phospholipase B-like 2 (PLBL2) from levels detected in initial samples. Tests confirm that antibodies used in AAE columns are broadly reactive, showing 55–92% coverage of HCPs from Chinese hamster ovary (CHO) cells with coverage percentage changing based on the method used.
Until recently, the concern over product-related proteins discouraged researchers from using MS for HCP detection. But AAE technology has enabled a robust MS workflow. First, AAE fractionation isolates sample HCP content; then, eluate undergoes tryptic digestion and MS injection. After that, researchers can analyze acquired spectra. This workflow can improve MS sensitivity and utility significantly. When asked whether MS could detect a specific HCP, analysts once assumed that a broadly reactive antibody did so. With an AAE–MS approach, analysts now can say definitively which copurifying “hitchhiker” proteins persist beyond purification.
An AAE–MS workflow can bolster HCP analytics at several critical junctures in biologics development. Before investigational new drug (IND) submission, AAE–MS steps can ensure that an HCP ELISA is fit for purpose. Moving into phase 2 clinical trials, it can drive characterization studies. But most important is that the AAE–MS approach can paint a complete picture of HCP content in drug substance. Learning about problematic proteins early in product development ultimately can reveal critical information about a drug’s stability and how that might affect patient safety.
Questions and Answers
How much sample does AAE fractionation require? Samples should have at least 100 µg of HCP. The requisite amount of drug substance depends on the stage of the purification process.
Do you recommend performing AAE–MS coverage on upstream harvest and final drug substance? It is important to test samples from both. Upstream analysis can reveal whether antibodies in your kit are broadly reactive to all or most potential HCPs. Downstream analysis identifies what HCPs ended up in a final drug substance. Having all that information enables effective risk assessment and decision-making regarding process changes.
Can AAE–MS testing quantify HCPs? Yes, we spike samples with standard proteins and then quantify the known and unknown HCPs based on those reference standards.
How do you deal with copurifying HCPs found in drug substance? Cygnus has tools for determining whether such proteins are present. If they are, then process changes must be made, and MS data such as isoelectric point (pI) and molecular weight can be passed to process development engineers.
More Online
The full presentation of this webcast can be found on the BioProcess International website at the link below.
You May Also Like