Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
May 13, 2020
Date: May 13, 2020
Duration: 20 Min
Sponsored by Bio-Rad
This webcast features: William H. Rushton, Process Chromatography Support Scientist, Bio-Rad Laboratories
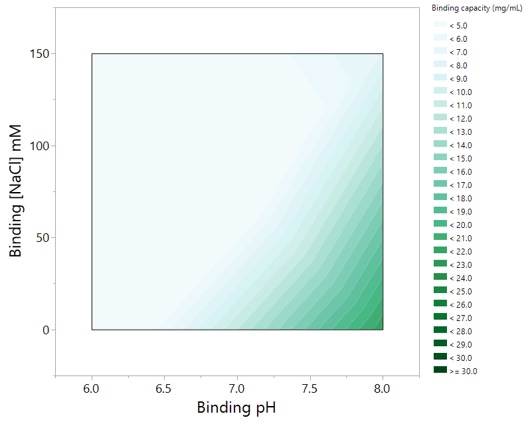
Viral clearance studies are part of a multifaceted approach to ensure the safety of biopharmaceutical products. In order to prevent costly changes to a manufacturing process, it is important to assess each operation unit for its efficiency on the removal or inactivation of adventitious agents early on during downstream process development.
A design of experiments (DOE) approach was utilized in this case study to investigate the effect of buffer pH and conductivity on the removal of various product- and process-related impurities by an anion-exchange (AEX) mixed-mode chromatography resin. Results from these studies have offered insights on the interactions between the resin and minute virus of mice (MVM) and xenotropic murine leukemia virus (XMuLV). A ceramic hydroxyapatite medium and a high-resolution cation-exchange (CEX) resin were also evaluated under typical conditions and will be presented in this webcast.
Overview:
Understand the interaction(s) between a model virus and chromatography resins
Evaluate the effect of critical process parameters
Assess the design space and optimal process condition
Use a pragmatic strategy to ensure viral safety in downstream processing
Watch the recorded webcast now.
You May Also Like