Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
November 10, 2014
Sponsored by Gyros
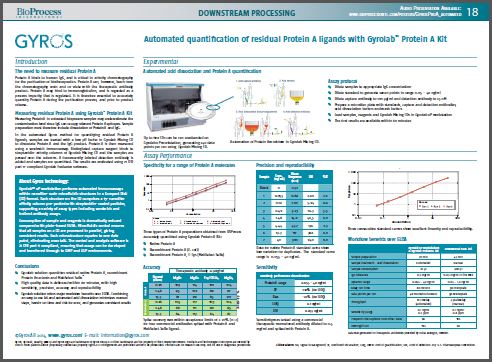
The Gyrolab™ immunoassay platform is well established for automating bioanalytical assays at nanoliter-scale in discovery, pre-clinical, clinical and bioprocess environments. In this presentation, we will show a new kit developed by Gyros using proprietary reagents and technology for the quantification of residual Protein A in bioprocess samples typically from downstream purification. The kit is comprised of labeled anti-Protein A antibodies, buffers for acid dissociation of IgG from Protein A and a Gyrolab mixing CD that automates the acid dissociation and analytical steps associated with the immunoassay in minutes. The combination of automated acid dissociation and assay processing offers workflow advantages over methods, such as ELISA, residual Protein A quantification. In addition to automation, the Gyrolab Protein A kit provides a robust and sensitive assay that is tolerant of several IgG subtypes as well as Fc-fusion proteins. Recovery of spiked protein A in samples containing 0.5 mg/mL of commercially available drugs was within ±20% of nominal concentration. Assay sensitivity and robustness have been assessed, with an LLOQ value of 0.1 ng/mL and typical inter-replicate CV percentages less than 10%. Overall the Gyrolab Protein A kit is a robust, sensitive and automated method to assess residual Protein A in bioprocess samples from downstream purification.
You May Also Like