Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Gyros
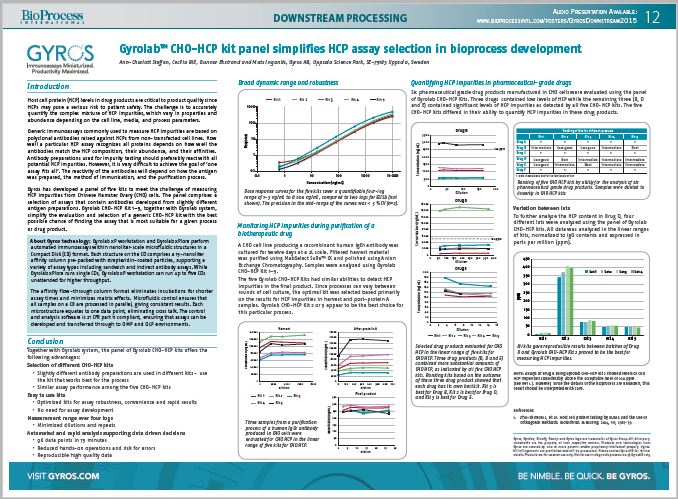
Host cell protein (HCP) levels in drug products are critical to product quality since HCPs may pose a serious risk to patient safety. The challenge is to accurately quantify the complex mixture of HCP impurities, which vary in properties and abundance depending on the cell line, media, and process parameters.
Generic immunoassays commonly used to measure HCP impurities are based on polyclonal antibodies raised against HCPs from non-transfected cell lines. How well a particular HCP assay recognizes all proteins depends on how well the antibodies match the HCP composition, their abundance, and their affinities. Antibody preparations used for impurity testing should preferably react with all potential HCP impurities. However, it is very difficult to achieve the goal of ‘one assay fits all’. The reactivity of the antibodies will depend on how the antigen was prepared, the method of immunization, and the purification process.
Gyros has developed a panel of five kits to meet the challenge of measuring HCP impurities from Chinese Hamster Ovary (CHO) cells. The panel comprises a selection of assays that contain antibodies developed from slightly different antigen preparations. Gyrolab CHO-HCP Kit 1–5, together with Gyrolab system, simplify the evaluation and selection of a generic CHO-HCP kit with the best possible chance of finding the assay that is most suitable for a given process or drug product.
You May Also Like