Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by ATCC

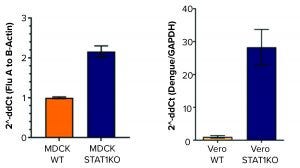
Figure 1: Model clinical virus production is increased in STAT1 KO cell lines with either (left) Influenza A or (right) Dengue II virus.
Antiviral vaccines are essential for preventing epidemic disease. However, production of them often is limited by low-yielding manufacturing processes. Similarly, the development of gene therapies is constrained during the production of adenoassociated virus (AAV) delivery platforms for gene transfer. To address the need for efficient viral synthesis to quicken the pace of bioproduction, ATCC used CRISPR/Cas9 genome-editing technology to develop STAT1 and BAX knockout (STAT1 BAX KO) cell lines to produce high-titer viral stocks. Three cell lines optimized for virus production were developed from parental cells commonly used in virus manufacturing. Those newly created cell lines can produce clinical viruses and AAVs at titers that are much higher than those of the parental cell lines, thereby providing an inexpensive method for biopharmaceutical companies to expand production.
Fill out the form below to read the complete technology review and learn about ATCC’s STAT1 BAX KO cell lines now.
You May Also Like